- Scholarli
- Posts
- Substitution reactions: A summary
Substitution reactions: A summary
A comprehensive summary of substitution reactions in Organic Chemistry 1
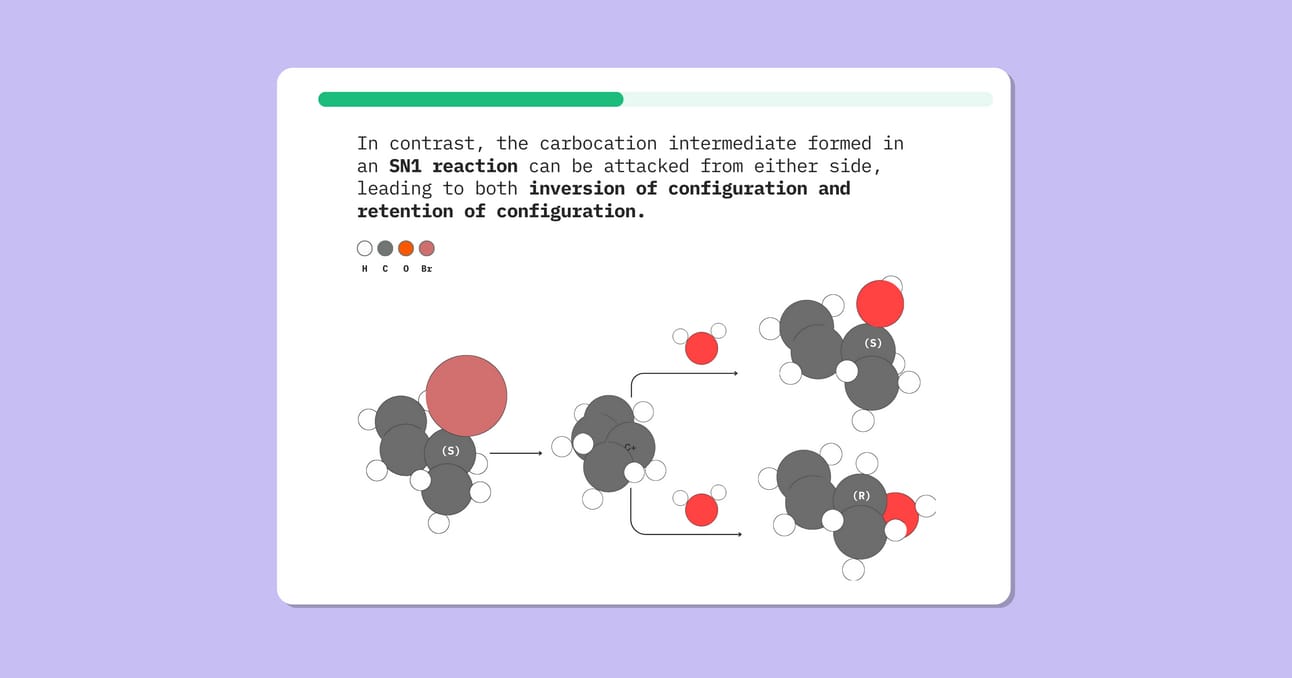
Table of Contents
Definition
Alkyl halides commonly undergo nucleophilic substitution (SN) reactions in which a nucleophile replaces the halogen.
SN1 vs SN2 reactions
There are two main types of nucleophilic substitution (SN) reactions, commonly referred to as SN1 and SN2. These labels denote the mechanisms by which these reactions occur.

Mechanisms
SN2 - Substitution Nucleophilic Bimolecular

In SN2 reactions, everything happens in one step. A nucleophile attacks the alkyl halide, causing the loss of a leaving group in a single rate-determining step. Therefore, Rate = k [Nucleophile] [alkyl halide]
SN1 - Substitution Nucleophilic Unimolecular

In SN1 reactions, the process occurs in two main steps: first, the leaving group exits, forming a carbocation; then, this carbocation is quickly attacked by a nucleophile. The first step of the reaction is the slowest and determines the overall rate because forming a carbocation, an unstable species, requires a lot of energy. Therefore, Rate = k [alkyl halide]
Stereochemistry of products
In an SN2 reaction, the nucleophile attacks from the opposite side of the leaving group, leading to an inversion of configuration.
In contrast, the carbocation intermediate formed in an SN1 reaction can be attacked from either side, leading to both inversion of configuration and retention of configuration.

Deciding Between SN1 and SN2
Factor 1: The electrophile
Compounds that form relatively stable carbocations generally react by the SN1 mechanism, while those that do not form stable carbocations react by the SN2 mechanism.
SN2 only

SN1 or SN2

SN1 only

Factor 2: The nucleophile
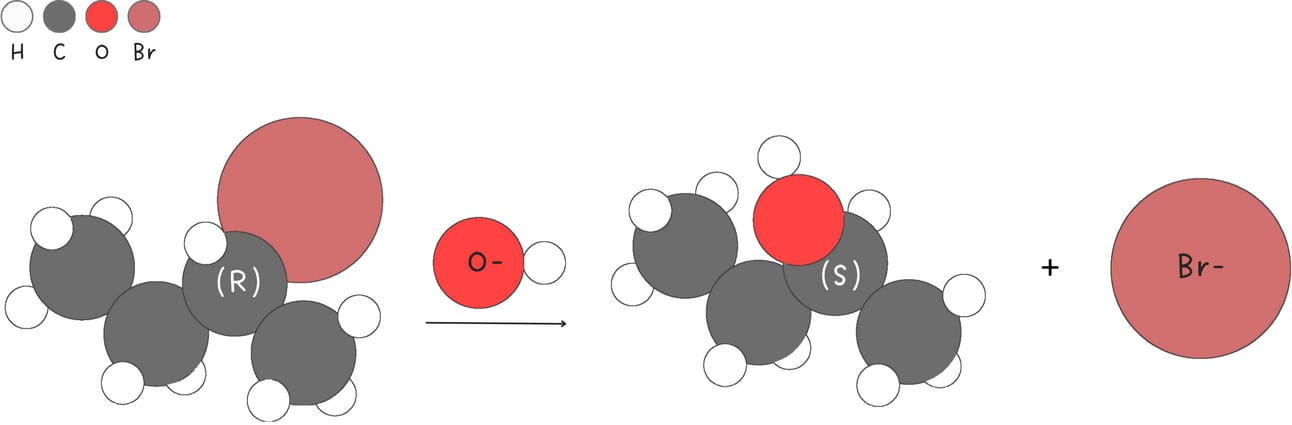
Strong nucleophiles favor SN2 reactions. Strong nucleophiles are typically negatively charged and highly polarizable due to size.

Weak nucleophiles do not favor SN2 reactions allowing SN1 reactions to compete successfully.

Factor 3: The leaving group
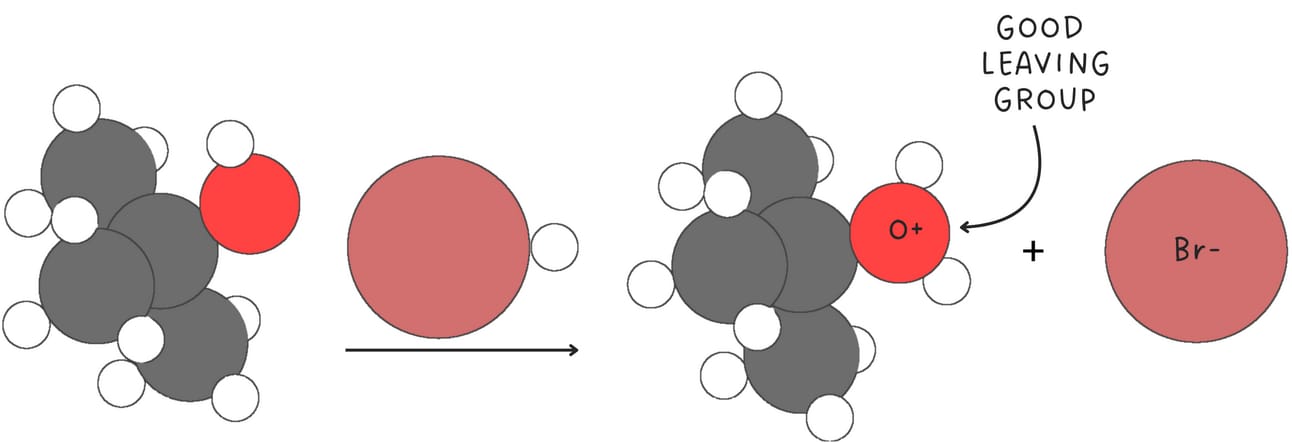
Both SN1 and SN2 reactions depend on the quality of the leaving group. If the leaving group is poor, neither reaction will proceed. SN1 reactions are generally more sensitive to the leaving group because the rate-determining step of an SN1 reaction involves the loss of a leaving group.
Some common good leaving groups are halides with iodine being the best leaving group.

Other good leaving groups include sulfonate ions which are resonance stabilized and water.

Bad leaving groups include:

Bad leaving groups can be converted to good ones when treated with a strong acid.
Factor 4: The solvent
Polar aprotic solvents favor SN2 reactions. Aprotic solvents do not contain N-H or O-H bonds.

Polar protic solvents contain N-H or O-H bonds and favor SN1 reactions. The solvents used in SN1 reactions also function as nucleophiles.

Reply