- Scholarli
- Posts
- Reactions of alkenes: A summary
Reactions of alkenes: A summary
A comprehensive summary of the reactions of alkenes in organic chemistry 1
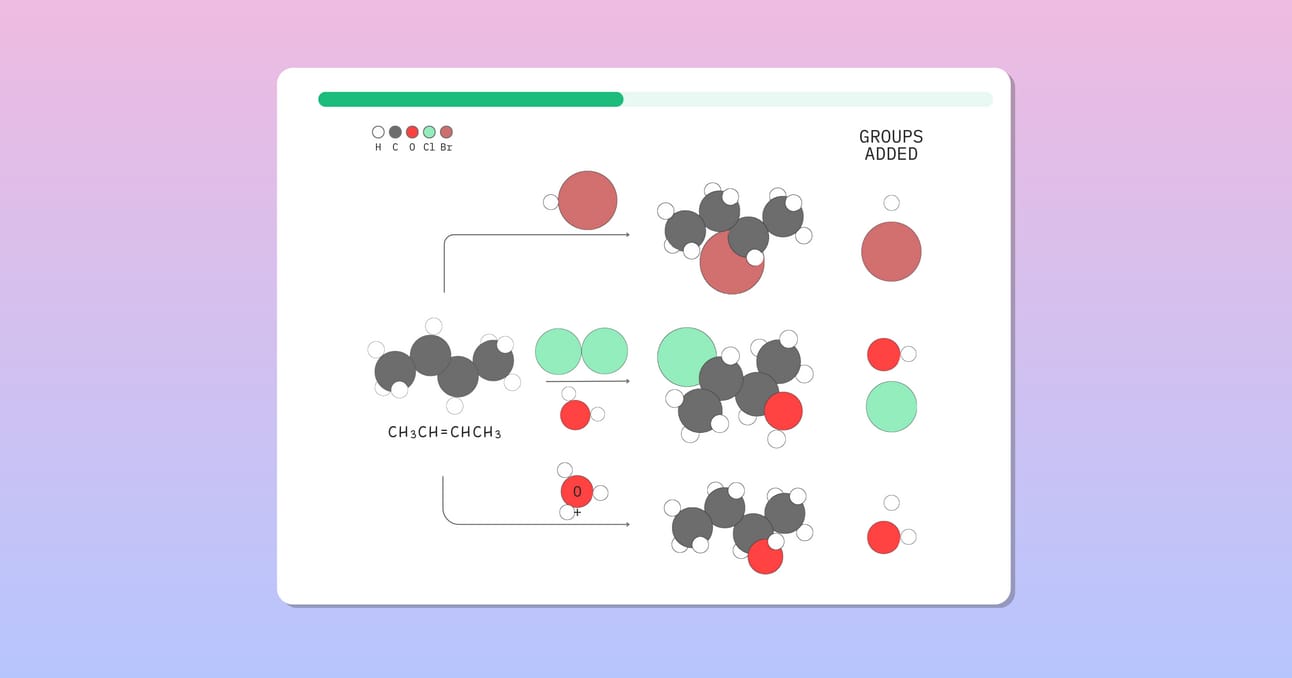
Table of Contents
Addition reactions
The reactions of alkenes are known as addition reactions. In addition reactions, two groups are added across the pi bond.
Terminology used in alkene reactions
Symmetrical vs unsymmetrical
Alkene structure can be described using two terms: symmetrical and unsymmetrical.
A symmetrical alkene has equal or identical substituents on each carbon of the double bond.
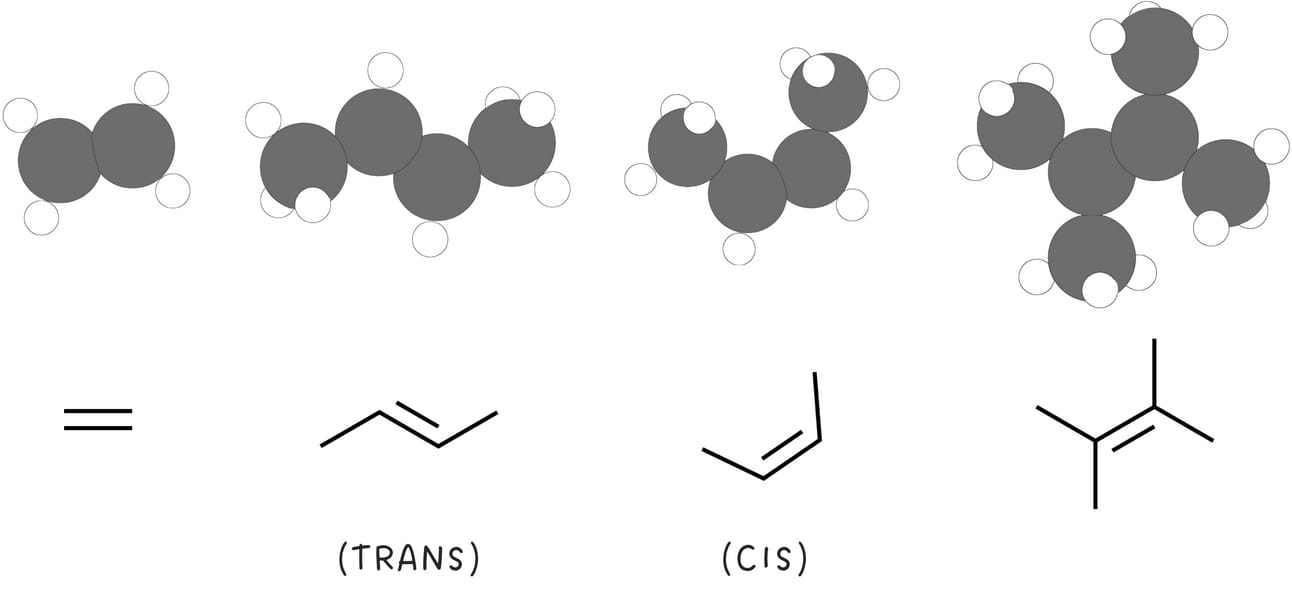
In an unsymmetrical alkene, the carbons of the double bond are not equally substituted.
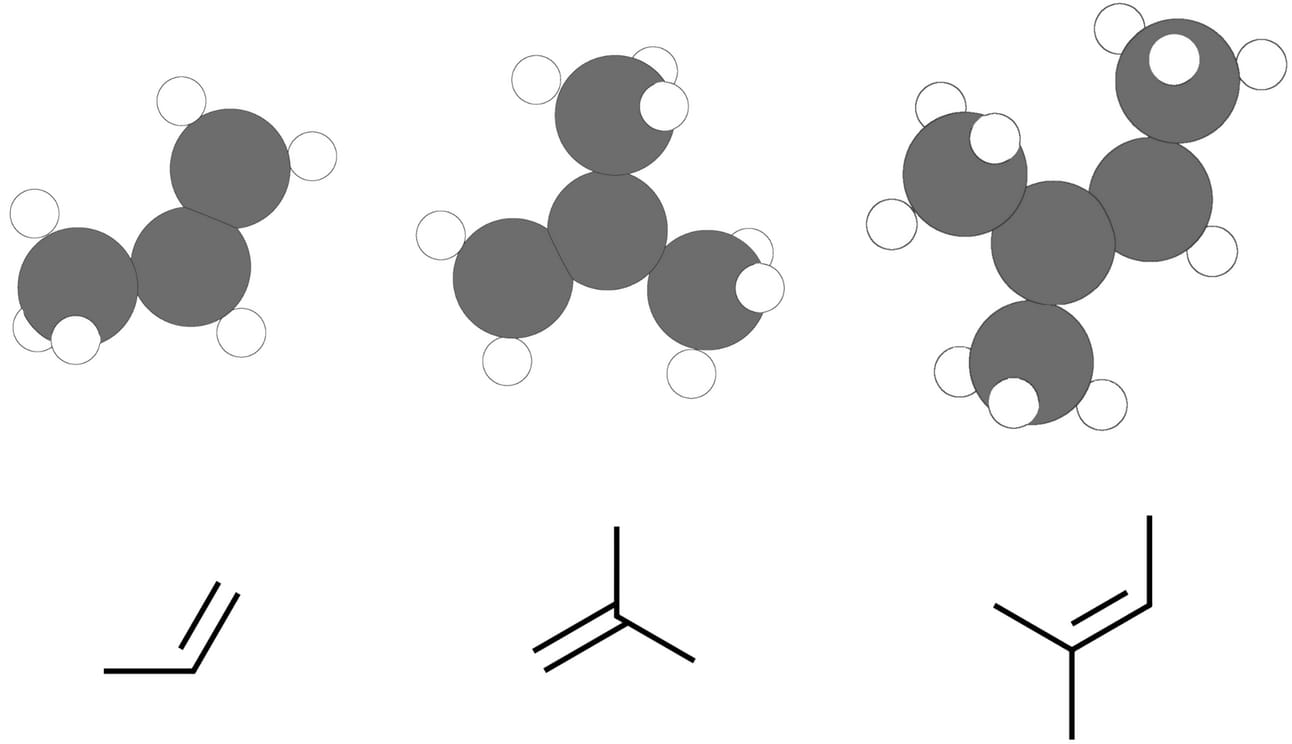
Markovnikov vs anti-Markovnikov
In a Markovnikov addition, the nucleophile adds to the more substituted carbon of an alkene. In an anti-Markovnikov addition, the nucleophile adds to the less substituted carbon.
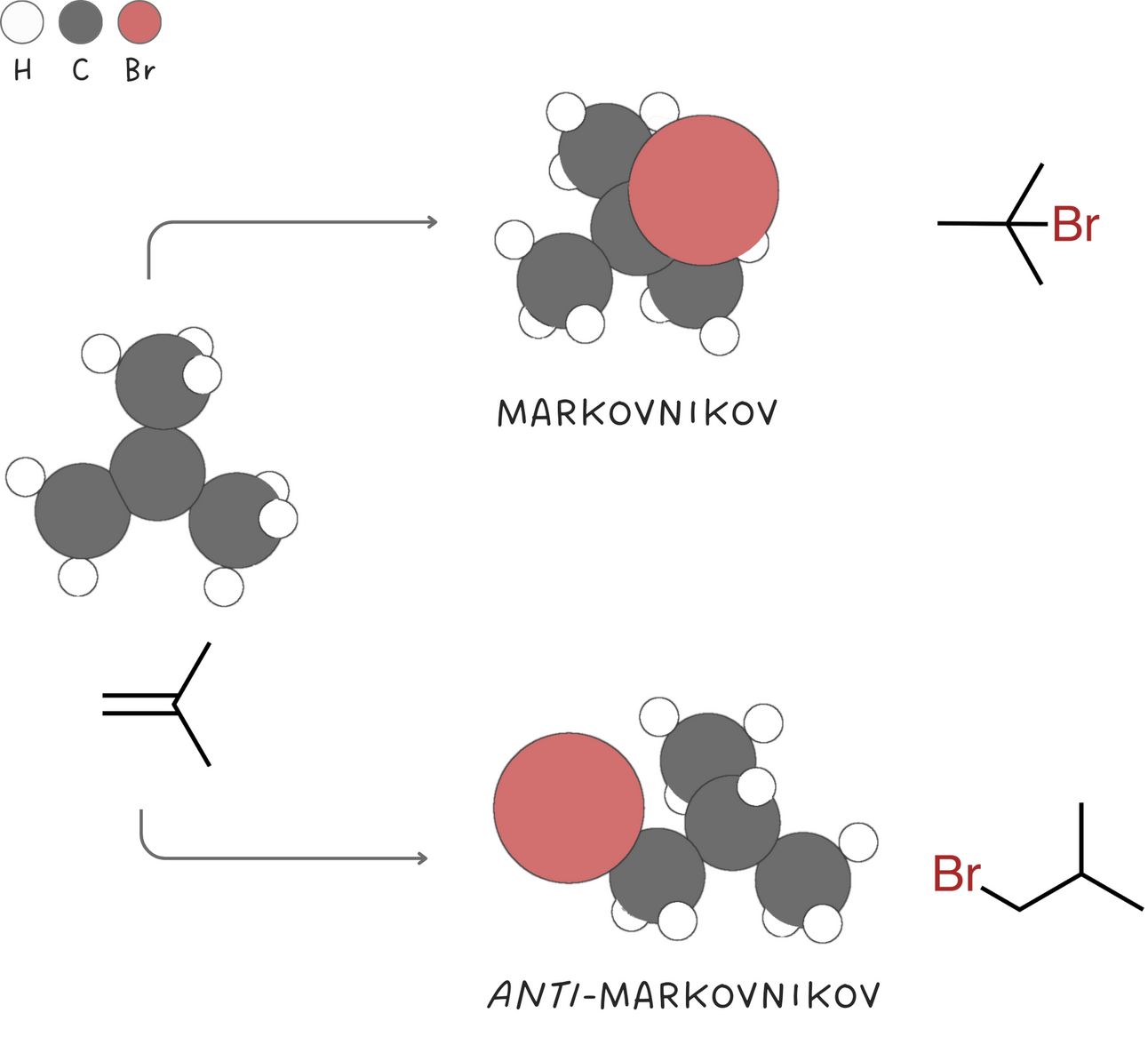
Unsymmetrical alkenes form either the Markovnikov or the anti-Markovinkov addition product, but not both. As such these reactions are said to be regioselective when only one of two possible pathways is preferred.
Syn vs anti addition
Syn addition refers to the addition of two substituents to the same side (or face) of a double bond. In contrast, anti addition involves the addition of two substituents to opposite sides (or faces) of the double bond.
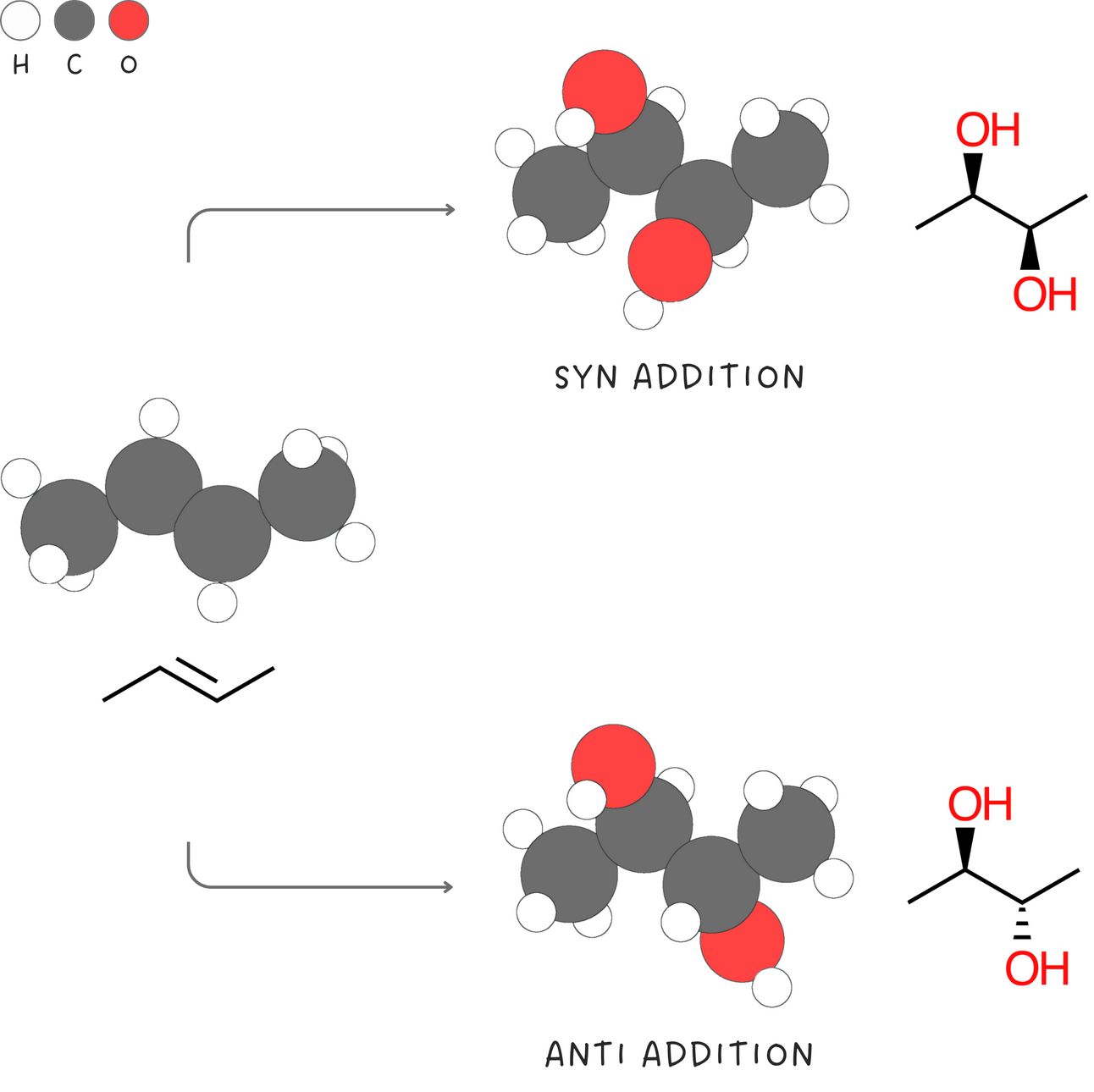
Reactions that occur only through anti addition or only through syn addition are said to be stereoselective. Reactions that form both syn and anti addition products are not stereoselective.
Reactions of alkenes
The reactions of alkenes can be grouped into four main categories based on the reaction pathway:
Carbocation pathway
Radical pathway
Three-membered ring pathway
Concerted pathway
Carbocation pathway
Reactions in this category involve two main steps: a proton transfer followed by a nucleophilic attack. In the proton transfer step, a proton adds to the alkene, generating a carbocation intermediate.
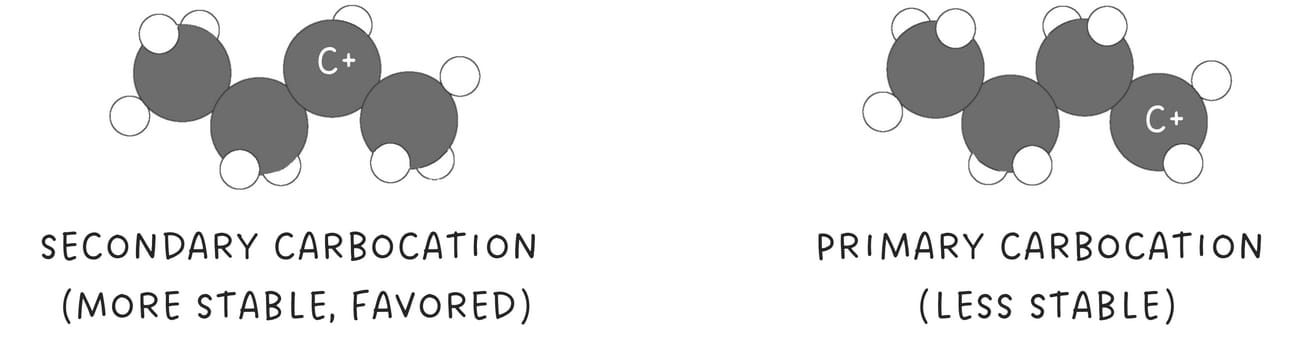
Then, a nucleophile attacks the more stable carbocation. The reaction follows Markovnikov's rule because the nucleophile adds to the more substituted carbon.
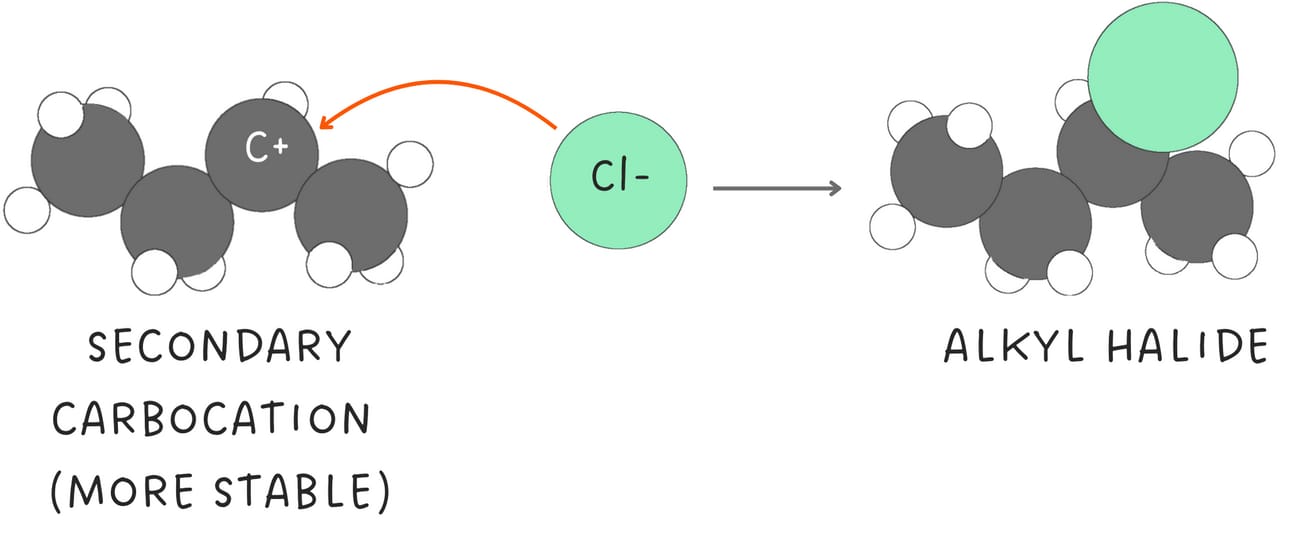
Additionally, since carbocations are formed, rearrangements are possible leading to the formation of multiple products.
Reactions in this category include:
Hydrohalogenation
Acid-catalyzed hydration
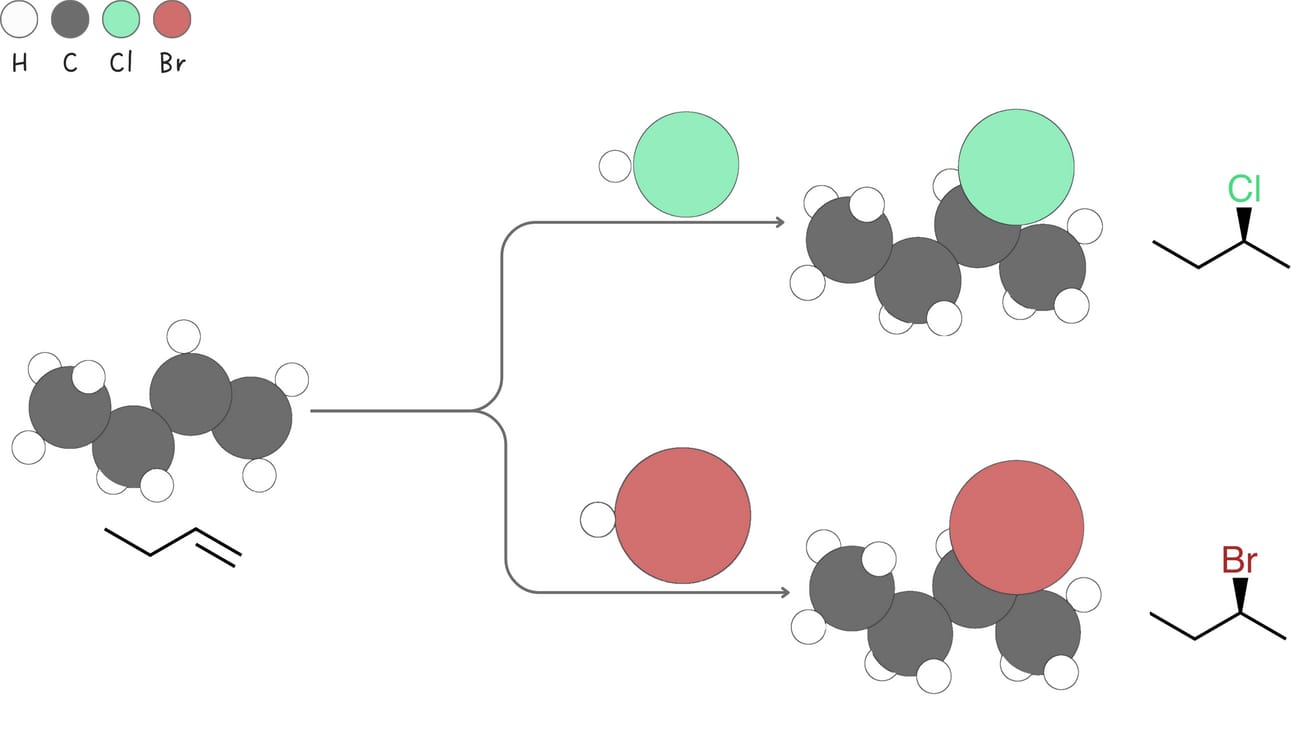
Hydrohalogenation (Addition of -H and -X)
Alkenes react with hydrogen halides (HX, like HCl or HBr) to form alkyl halides.
Regiochemistry: Markovnikov
Stereochemistry: A mixture of syn + anti
Rearrangements: Yes
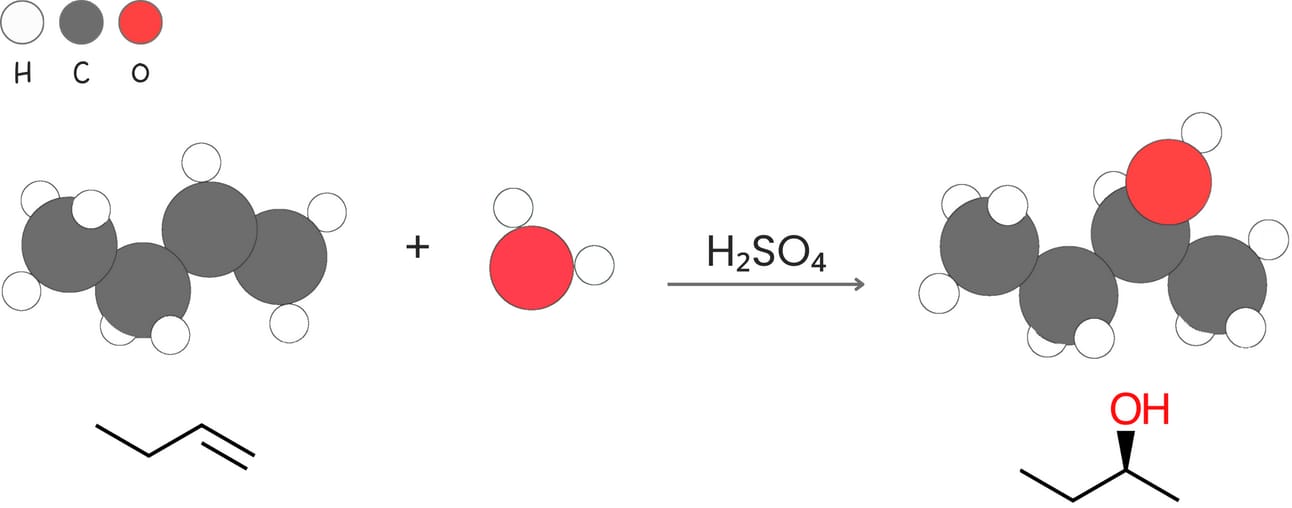
Acid-catalyzed hydration (Addition of -H and -OH)
Alkenes react with water in the presence of an acid catalyst such as H2SO4 to form alcohols.
Regiochemistry: Markovnikov
Stereochemistry: A mixture of syn + anti
Rearrangements: Yes
Radical Pathway
The primary reaction in this category is the radical addition of HBr. In the presence of peroxides, such as hydrogen peroxide (H2O2) or the commonly used meta-chloroperoxybenzoic acid (mCPBA), the addition of -H and -Br proceeds via an anti-Markovnikov addition.
Regiochemistry: anti-Markovnikov
Stereochemistry: A mixture of syn + anti
Rearrangements: No
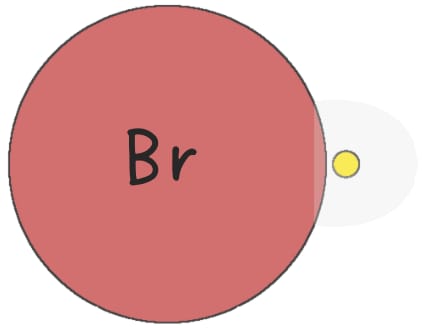
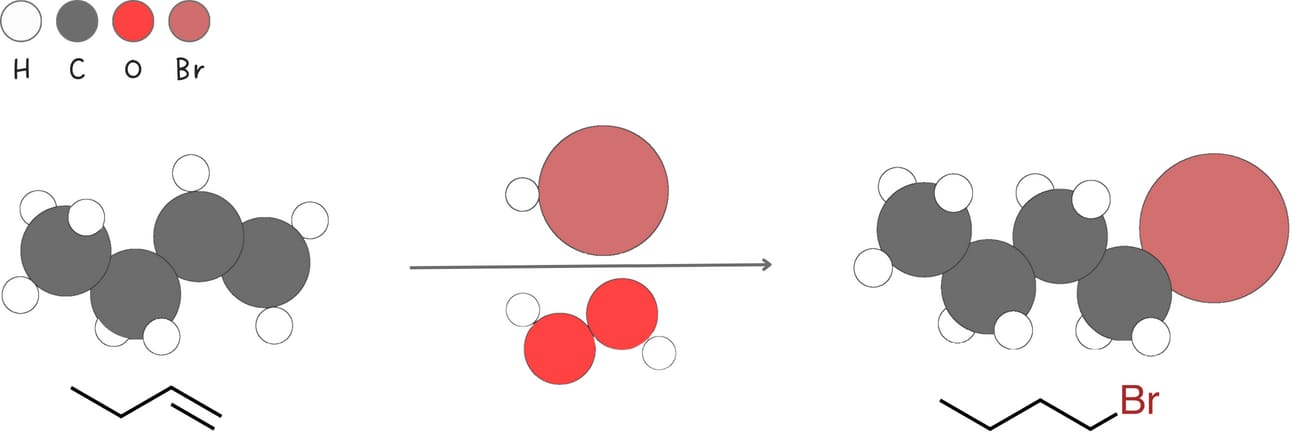
In the radical addition of HBr to an alkene, peroxides generate a bromine radical.
The bromine radical adds to the pi bond generating a carbon radical intermediate.
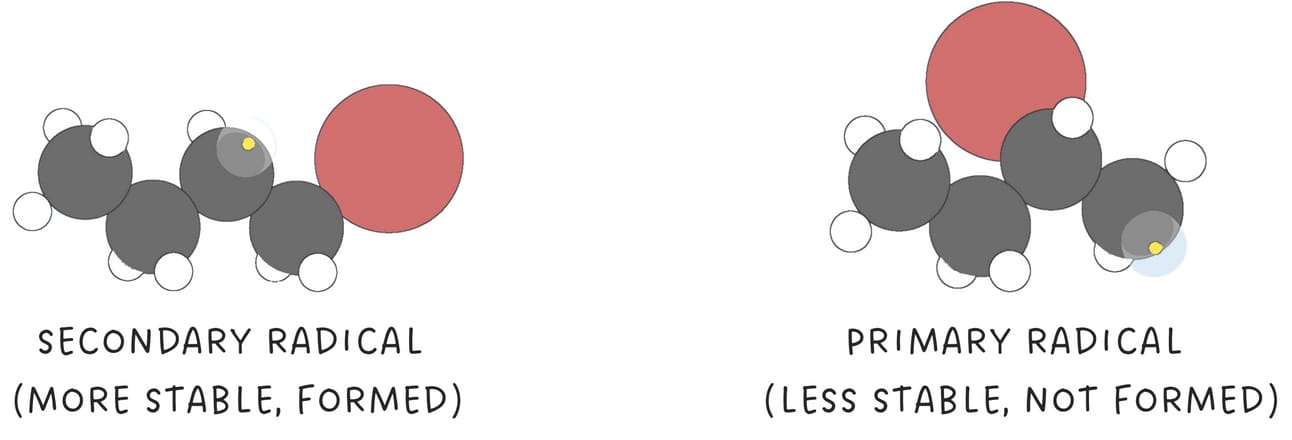
The observed anti-Markovnikov regioselectivity arises because the reaction proceeds via the most stable carbon radical intermediate.
Three-membered ring pathway
Reactions in this category involve a three-membered ring intermediate.
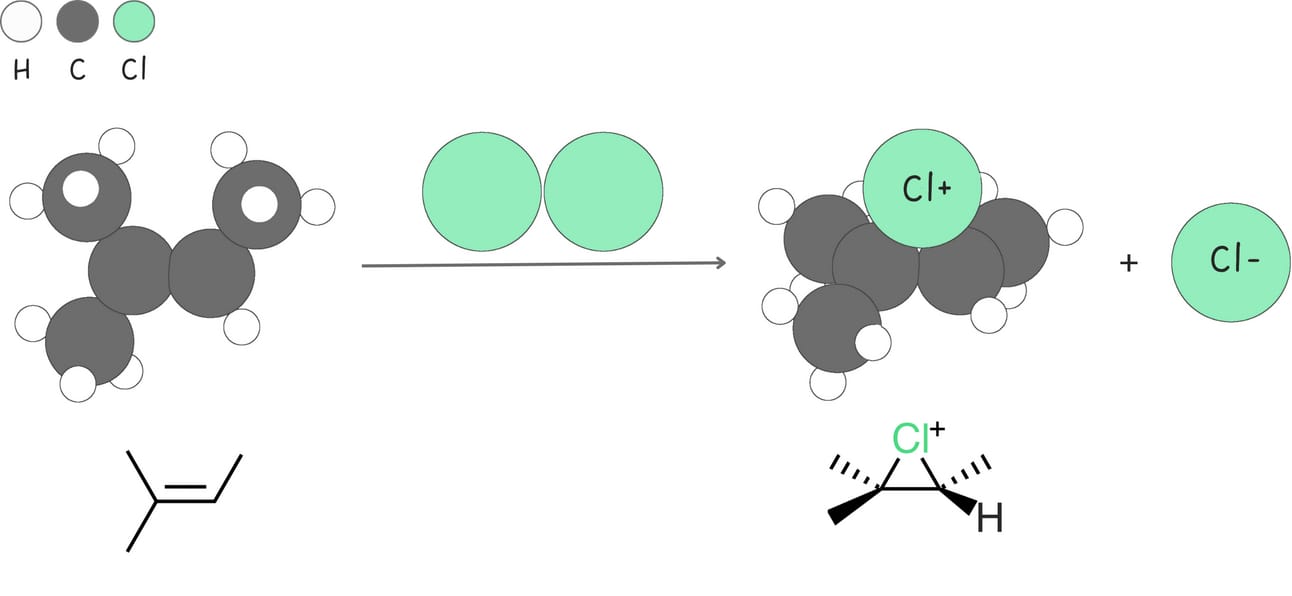
These reactions follow a Markovnikov-like pattern, where the nucleophile attacks the more substituted carbon of the three-membered ring from the backside, resulting in the formation of anti-addition products.
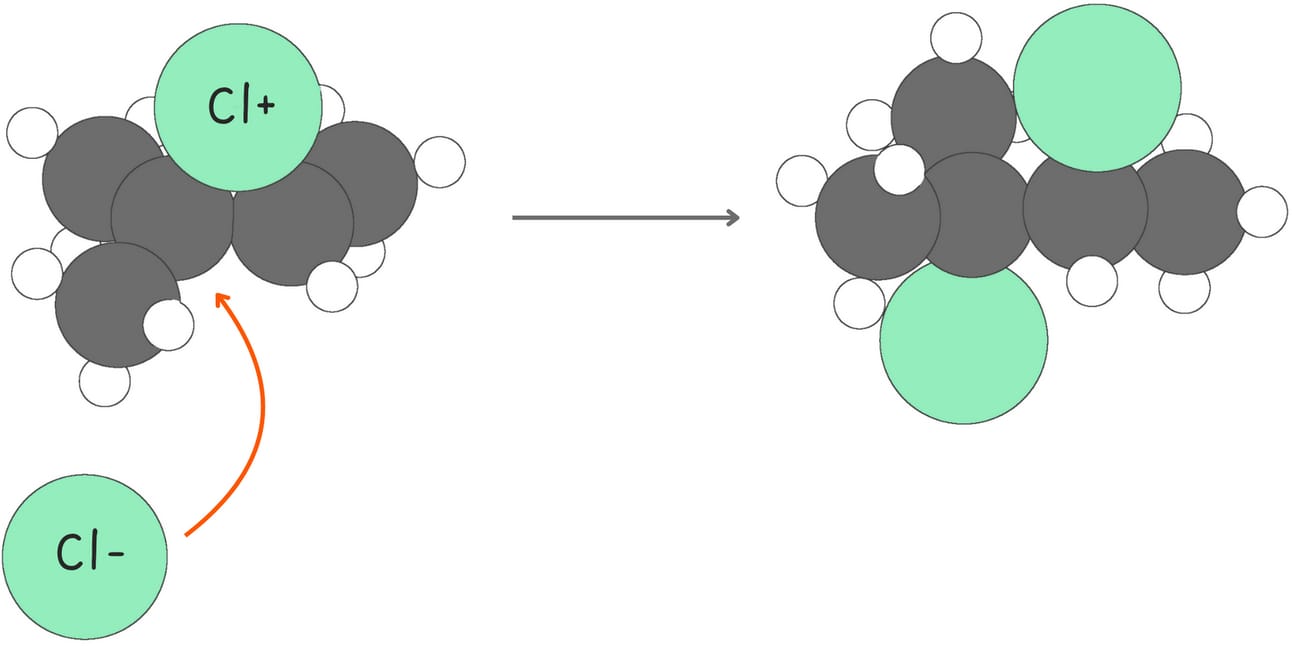
However, oxymercuration-demercuration is an exception to this rule. There is ample evidence indicating that demercuration occurs through a radical process, forming both syn and anti addition products.
Reactions in this category include:
Halogenation
Halohydrin formation
Anti-dihydroxylation
Oxymercuration-demercuration
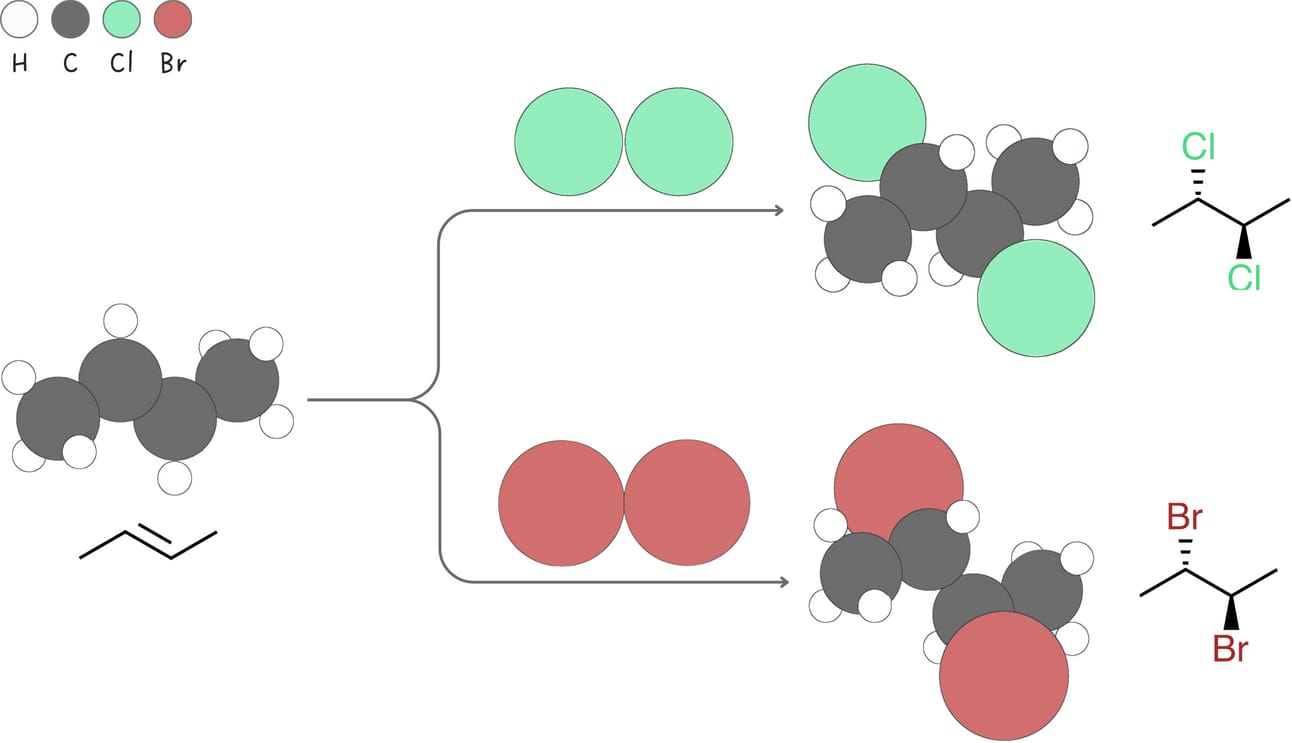
Halogenation (Addition of X2)
This reaction involves the addition of halogens such as Cl2 or Br2 across an alkene.
Regiochemistry: Markovnikov-like
Stereochemistry: anti
Rearrangements: No
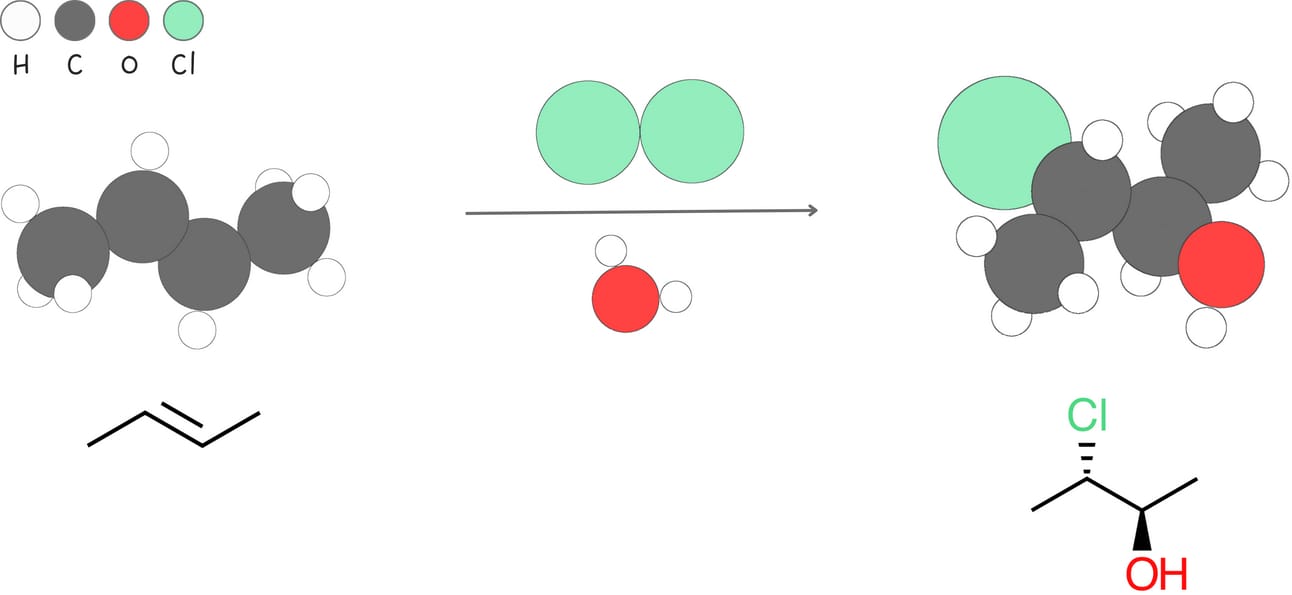
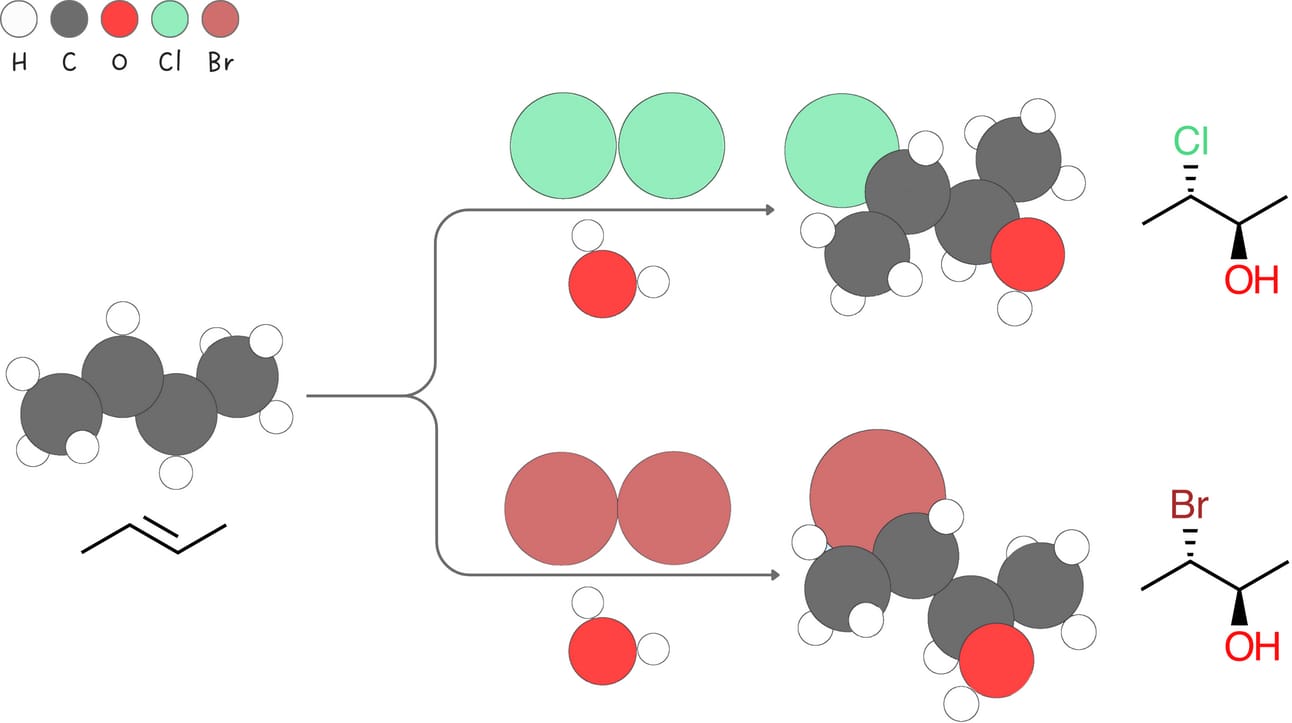
Halohydrin formation (Addition of -X and -OH)
This reaction adds a halogen, -X, and -OH to an alkene, forming a halohydrin.
Regiochemistry: Markovnikov-like
Stereochemistry: anti
Rearrangements: No
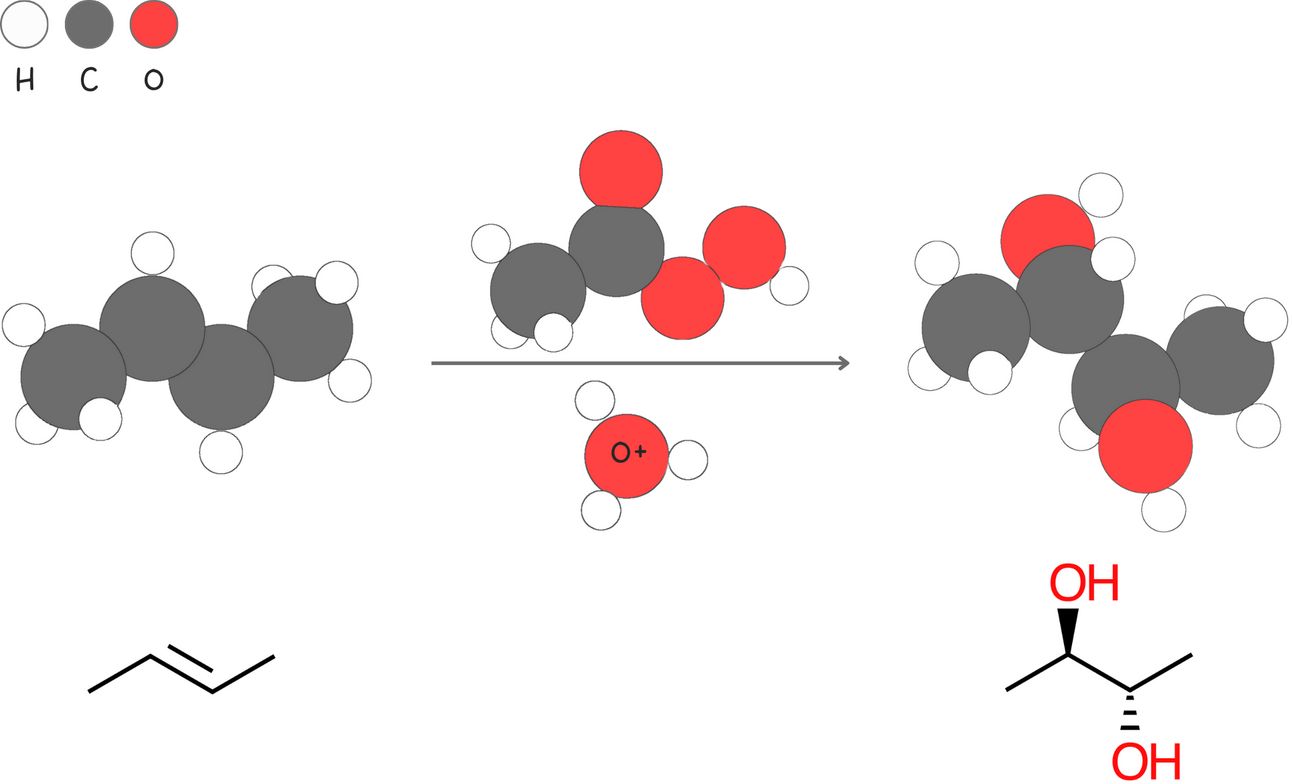
Anti-dihydroxylation (Addition of -OH and -OH)
This two-step reaction converts an alkene into an epoxide, followed by an acid-catalyzed ring opening. The net result is the anti addition of two -OH groups across the alkene.
Regiochemistry: Markovnikov-like
Stereochemistry: anti
Rearrangements: No
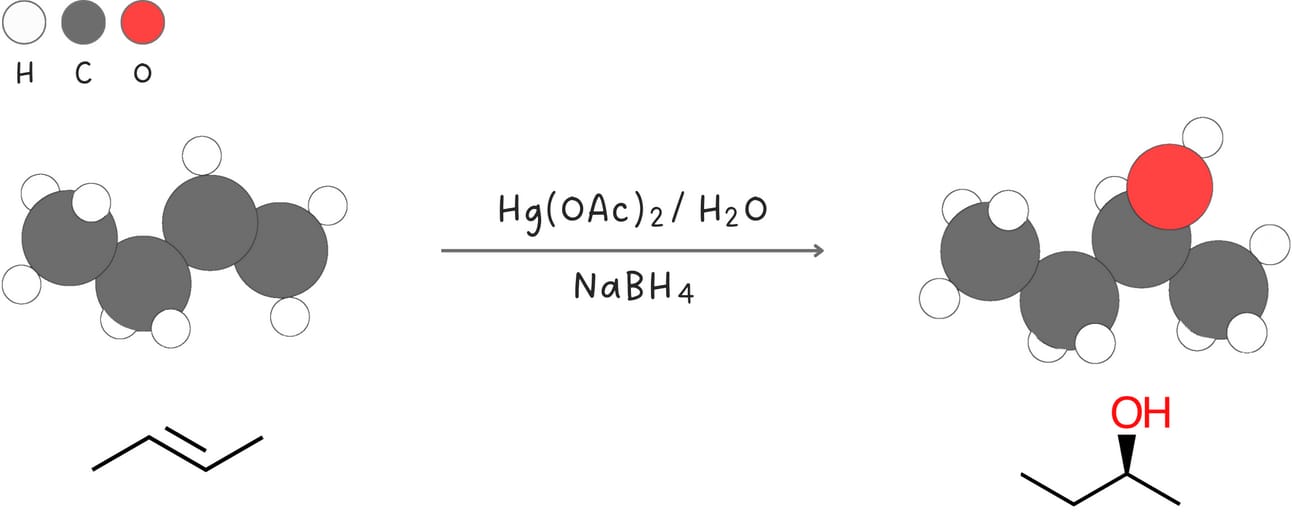
Oxymercuration-demercuration (Addition of -H and -OH)
This reaction adds -H and -OH across an alkene without carbocation rearrangements.
Regiochemistry: Markovnikov-like
Stereochemistry: anti
Rearrangements: No
Concerted pathway
In a concerted reaction, all bond-breaking and bond-forming processes occur simultaneously in a single step.
This is different from a stepwise mechanism, where intermediates are formed. In these reactions, two groups are added to the same side (or face) of the double bond in a concerted process, giving rise to syn addition products.
Reactions in this category include:
Hydrogenaton
Hydroboration-oxidation
Carbene formation
Syn-dihydroxylation
Epoxidation
Ozonolysis
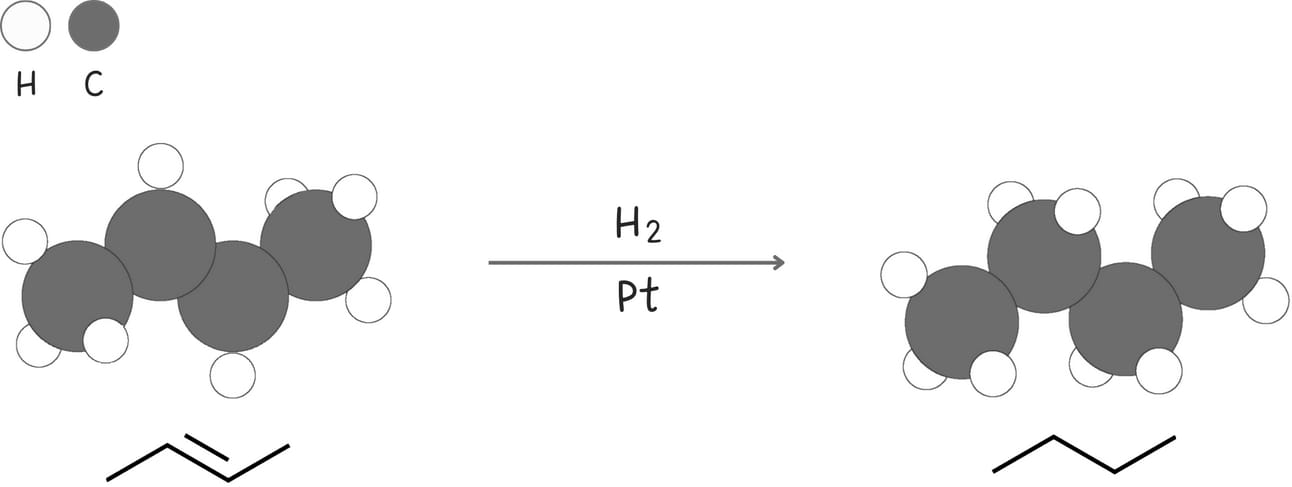
Hydrogenation (Addition of H2)
This reaction adds two hydrogen atoms across an alkene forming an alkane.
Regiochemistry: N/A
Stereochemistry: syn
Rearrangements: No
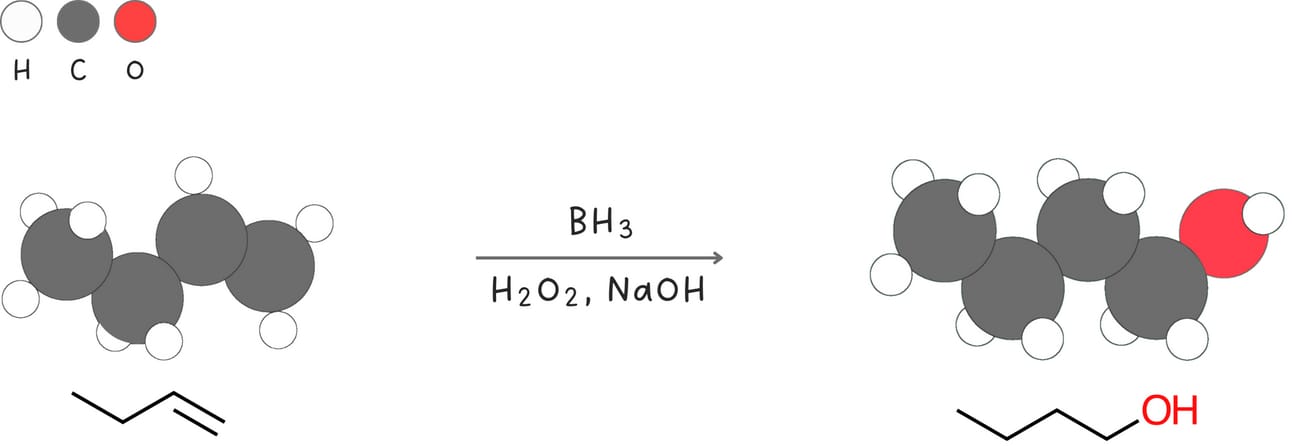
Hydroboration-oxidation (Addition of -H and -OH)
This reaction adds -H and -OH across an alkene forming an alcohol without any carbocation rearrangements.
Regiochemistry: anti-Markovnikov
Stereochemistry: syn
Rearrangements: No
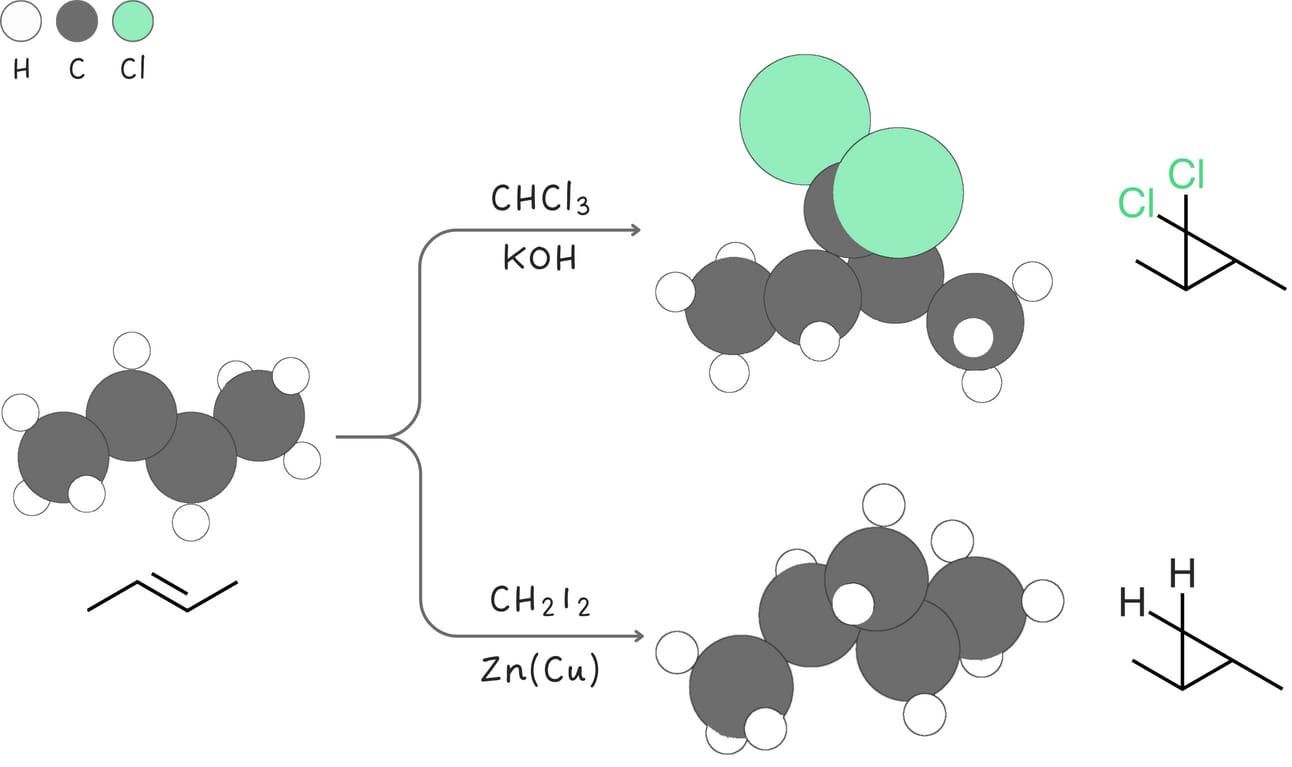
Addition of carbenes
Alkenes react with carbenes to yield cyclopropane.
Regiochemistry: anti-Markovnikov
Stereochemistry: syn
Rearrangements: No
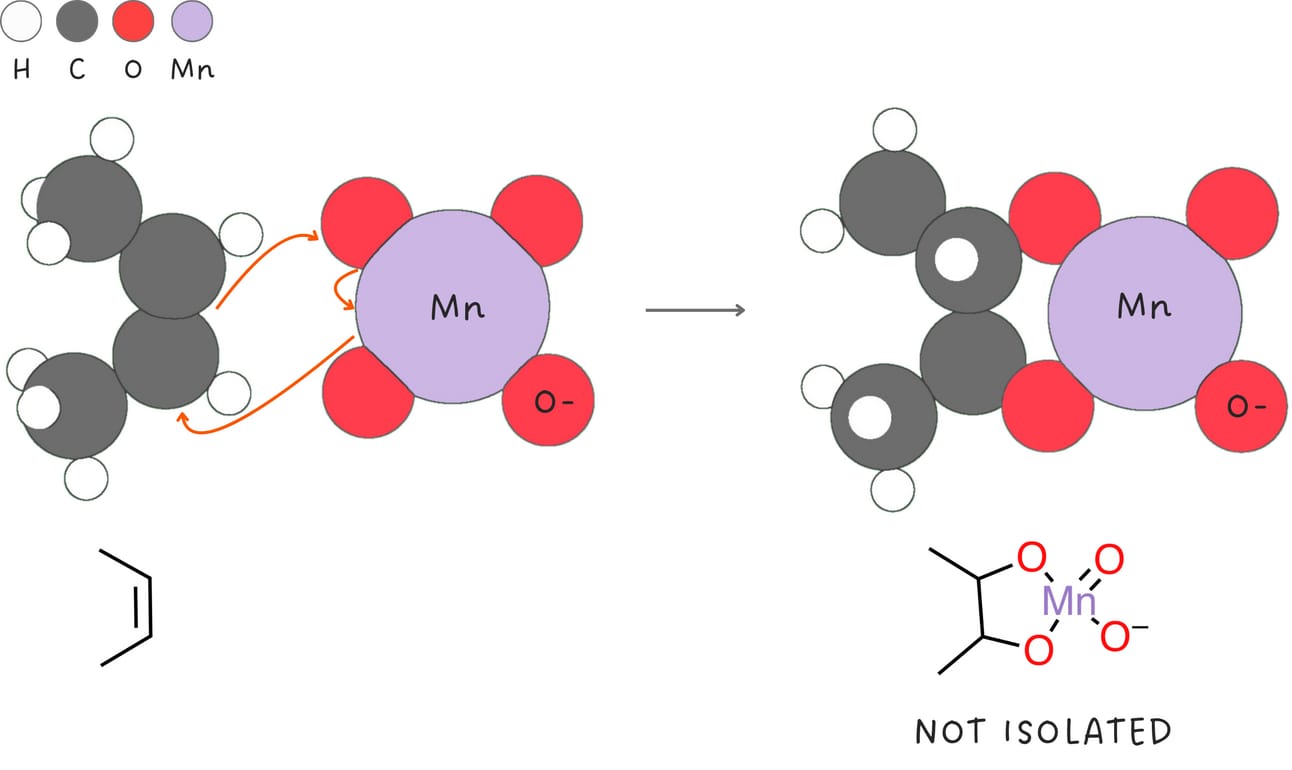
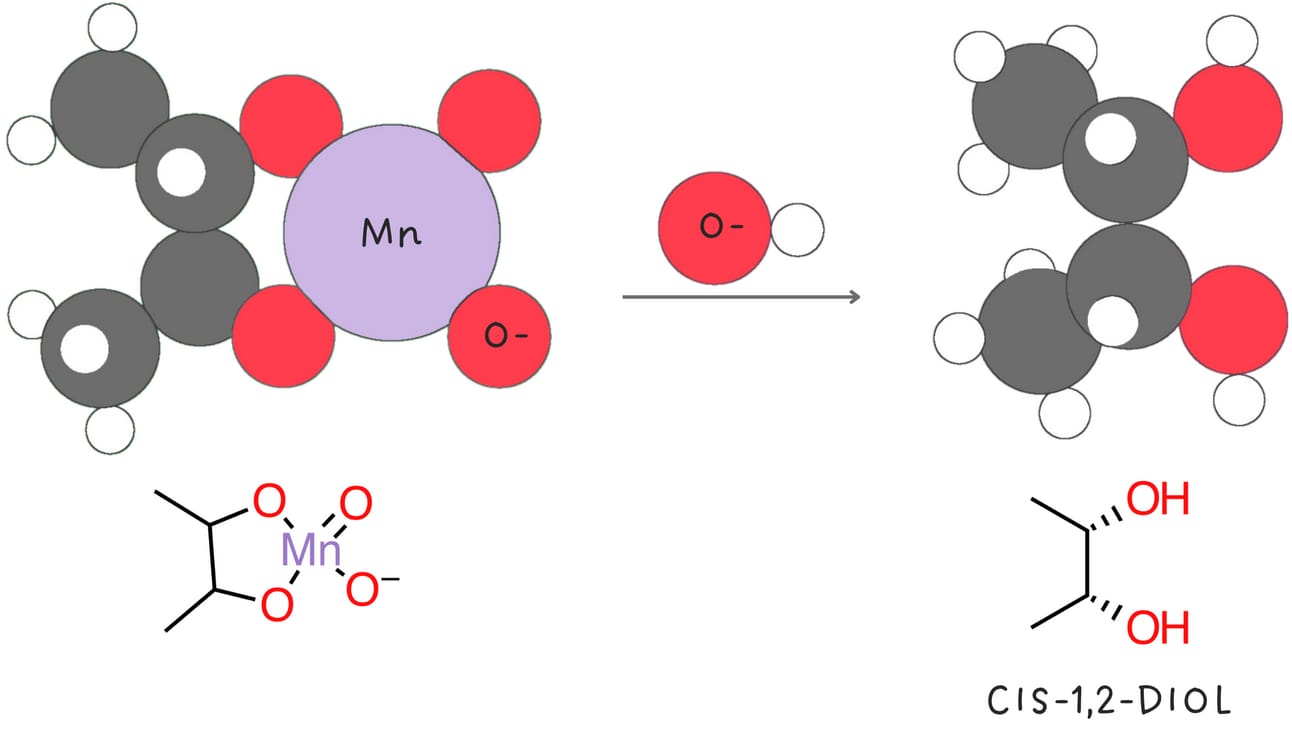
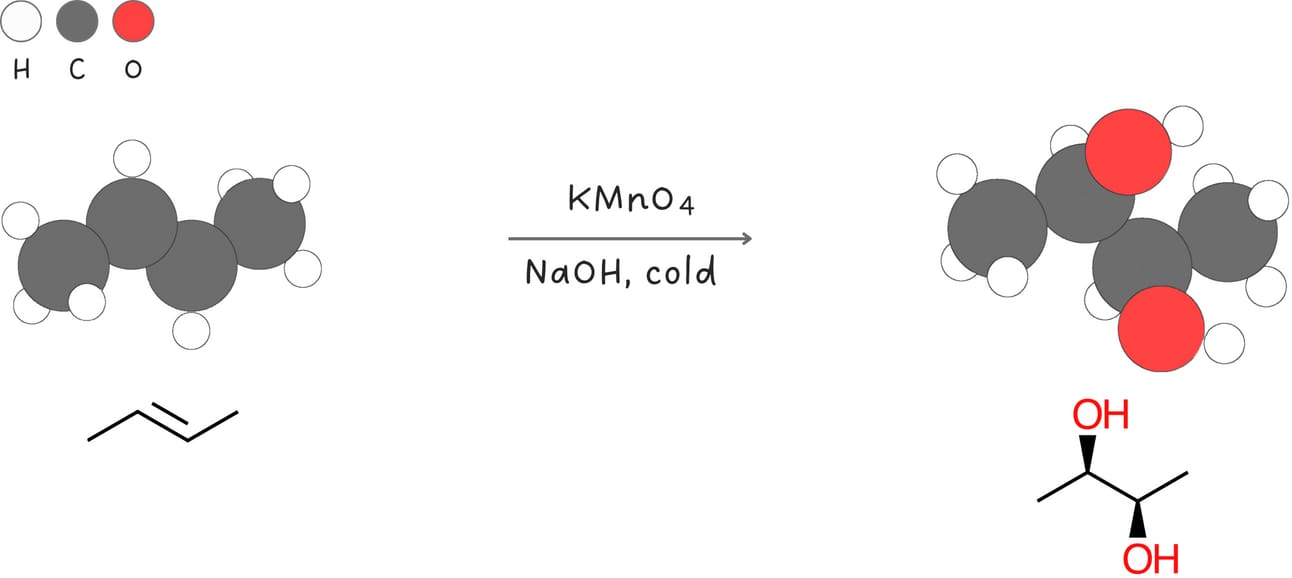
Syn-dihydroxylation (Addition of -OH and -OH)
This reaction adds two -OH groups across an alkene in a syn fashion.
Regiochemistry: N/A
Stereochemistry: syn
Rearrangements: No
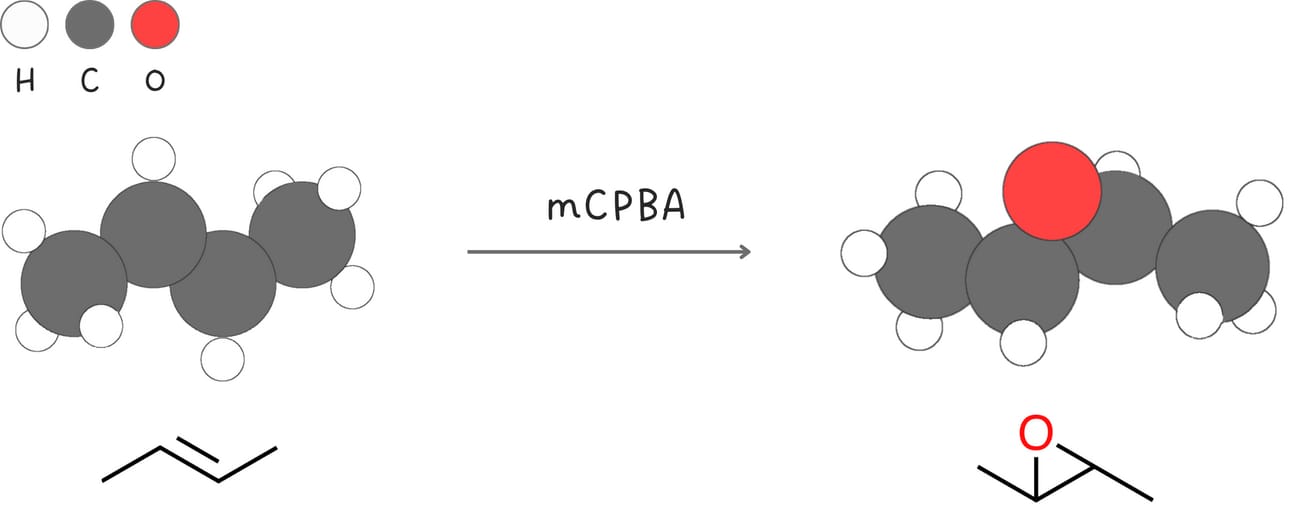
Epoxidation
Alkenes are oxidized in the presence of peroxyacids forming epoxides.
Regiochemistry: N/A
Stereochemistry: syn
Rearrangements: No
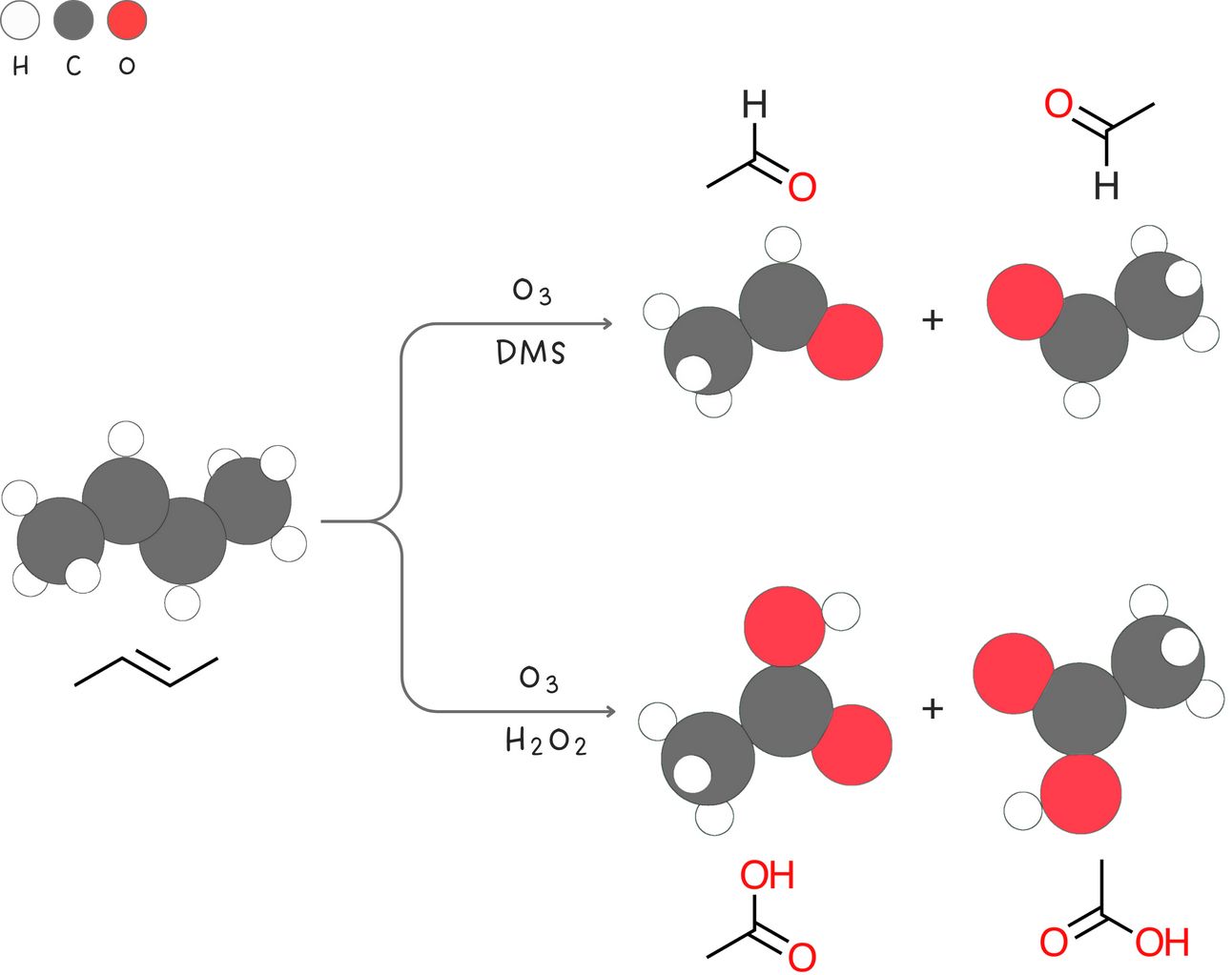
Ozonolysis
Powerful oxidizing agents such as ozone (O3) are powerful enough to cleave the carbon-carbon double of an alkene and produce two carbonyl compounds.
Regiochemistry: N/A
Stereochemistry: syn
Rearrangements: No
Reply