- Scholarli
- Posts
- Reactions of alkynes: A summary
Reactions of alkynes: A summary
A comprehensive summary of the reactions of alkynes in organic chemistry 1
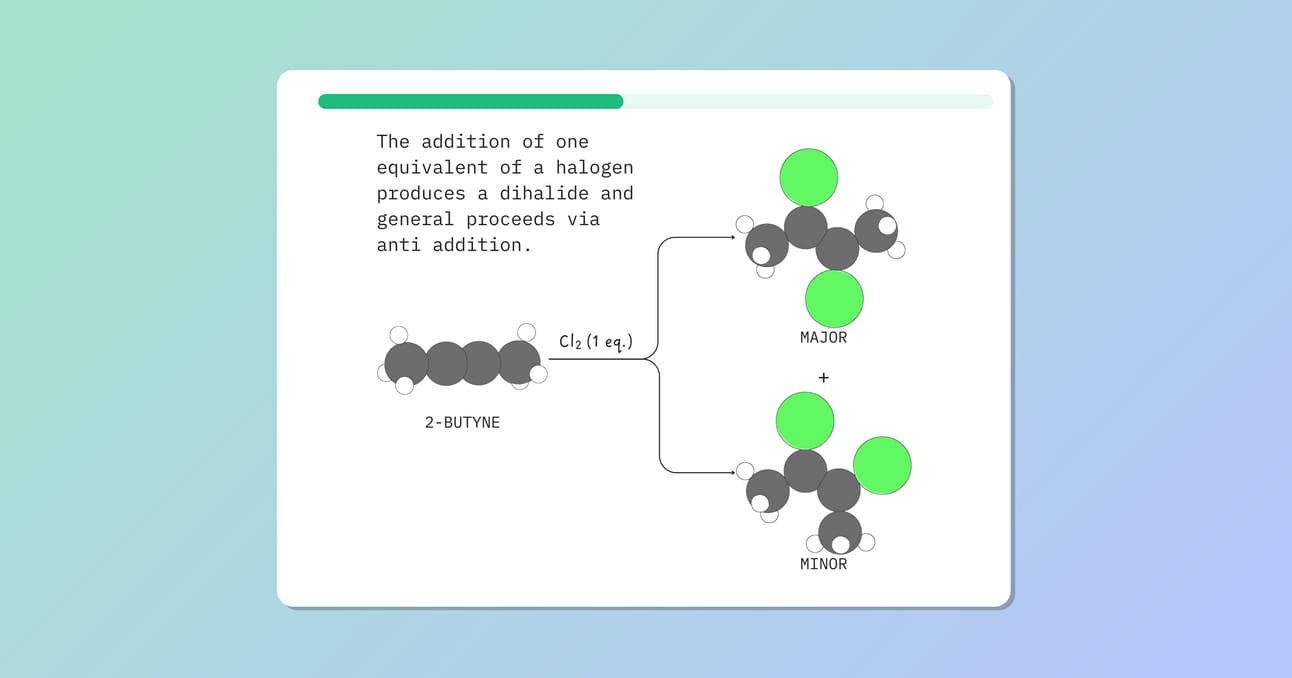
Table of Contents
Alkynes
Alkynes are hydrocarbons that contain at least one carbon-carbon triple bond. This triple bond consists of one sigma bond and two pi bonds.
Terminal alkynes vs internal alkynes
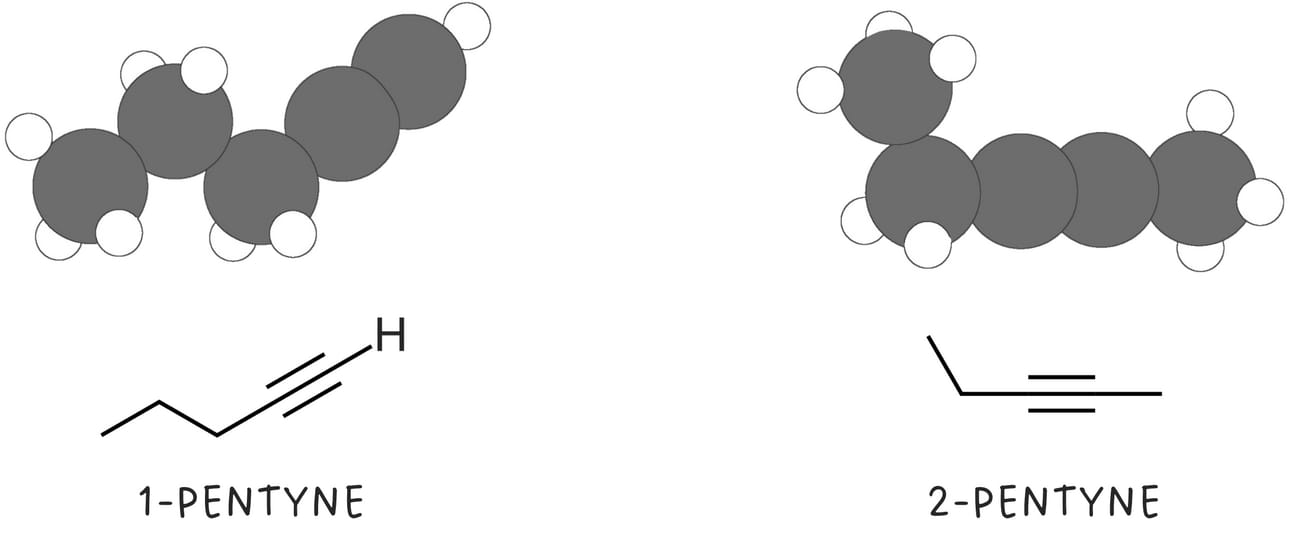
Terminal alkynes contain a triple bond at the end of the carbon chain. Typically, one hydrogen is bonded to one of the carbons of the triple bond.
General Formula: RC≡CH

Disubstituted or internal alkynes have a triple bond between two carbon atoms within the carbon chain.
General Formula: RC≡CR'
Addition reactions of alkynes
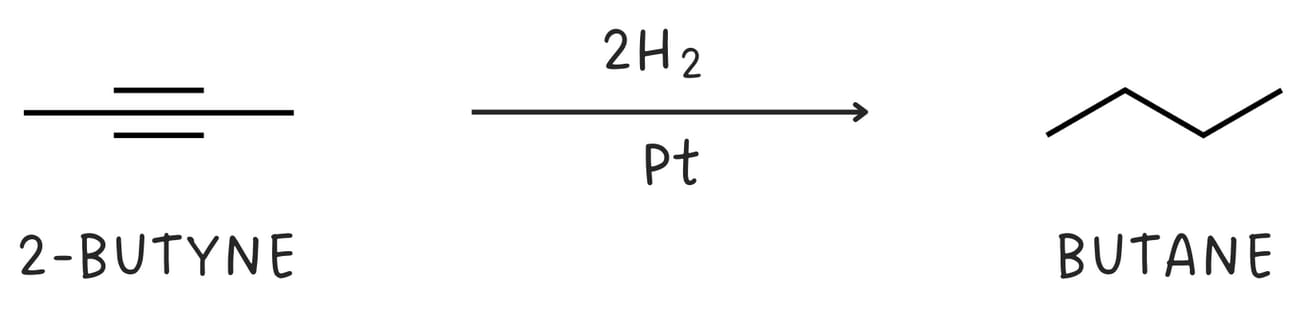
Reduction (Addition of H2)
Alkynes can be reduced to alkenes and alkanes. The complete reduction of alkyne yields an alkane.
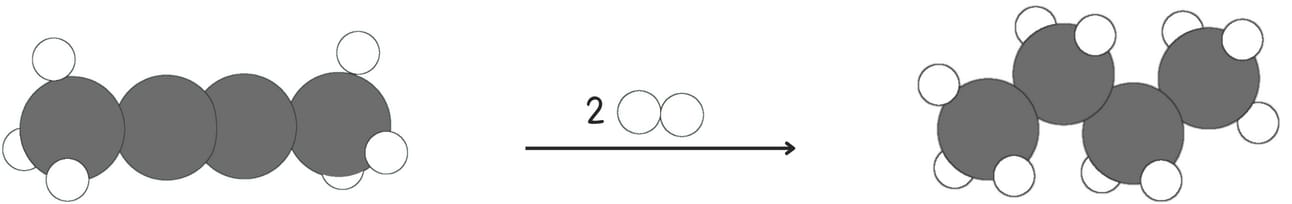
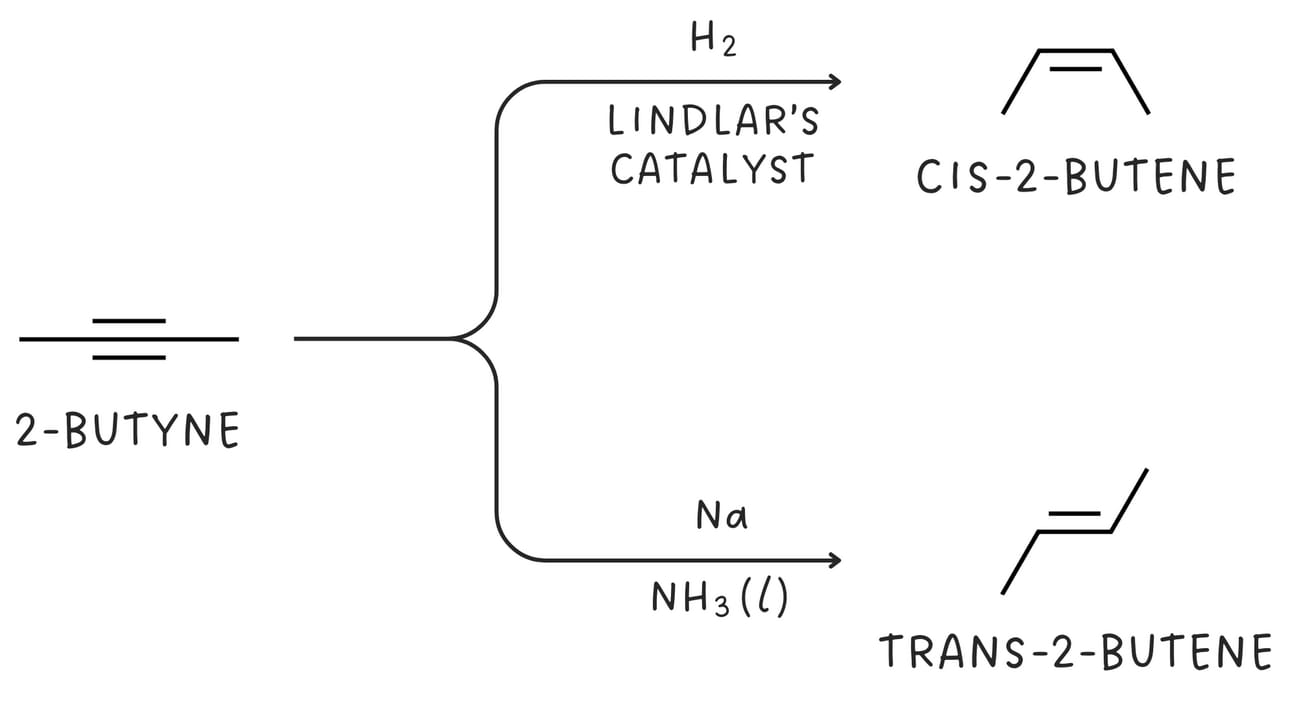
The partial reduction of alkyne yields an alkene.
Halogenation (Addition of X2)
Alkynes react with halogens like Cl2 or Br2. The addition of one equivalent of a halogen produces a dihalide and generally proceeds via anti addition.
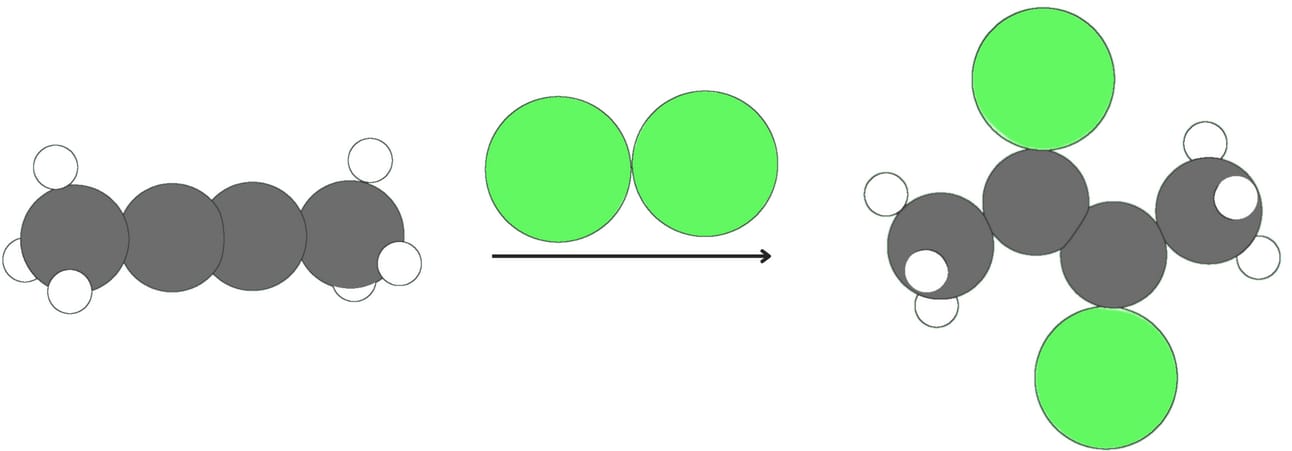
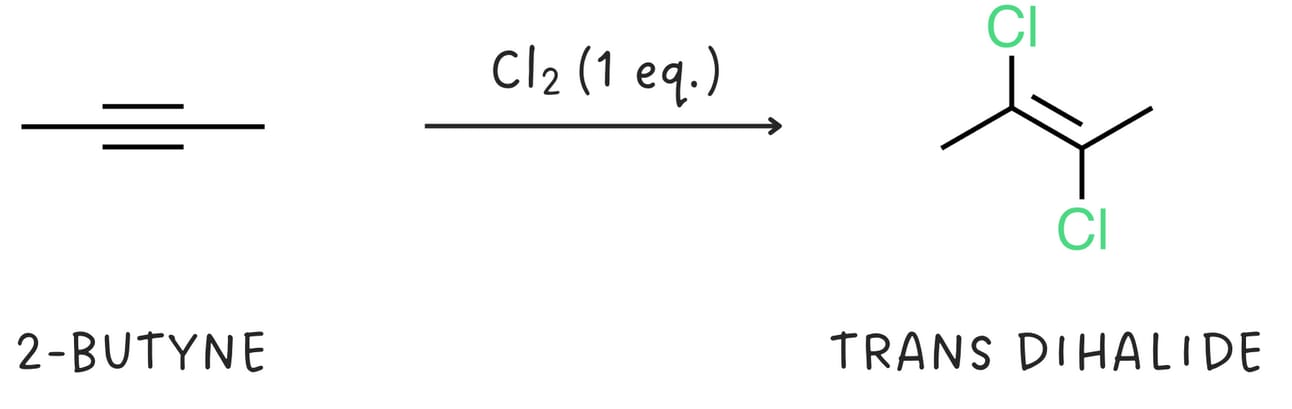
The dihalide can react with another equivalent of halogen to produce a tetrahalide.
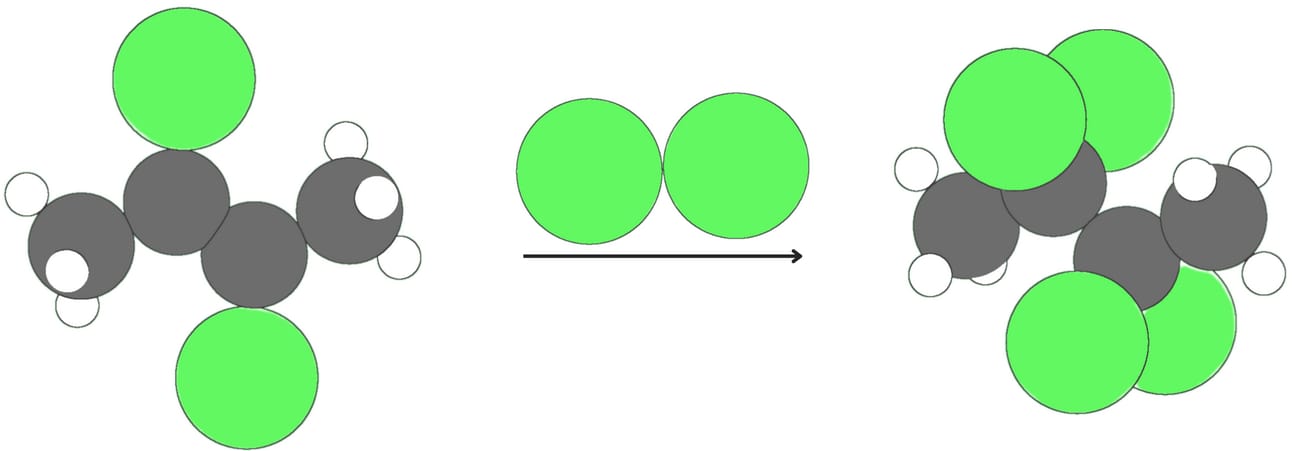
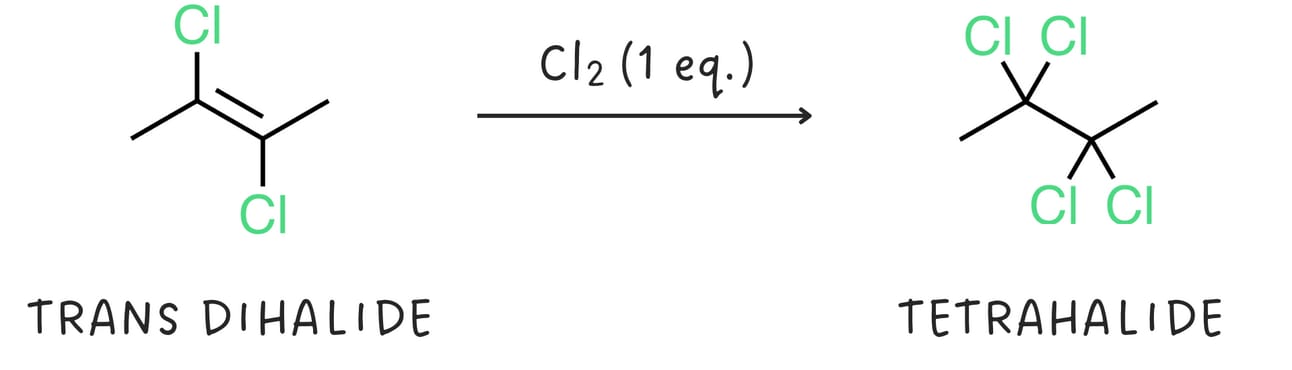
Hydrohalogenation (addition of -H and -X)
Regiochemistry: Markovnikov
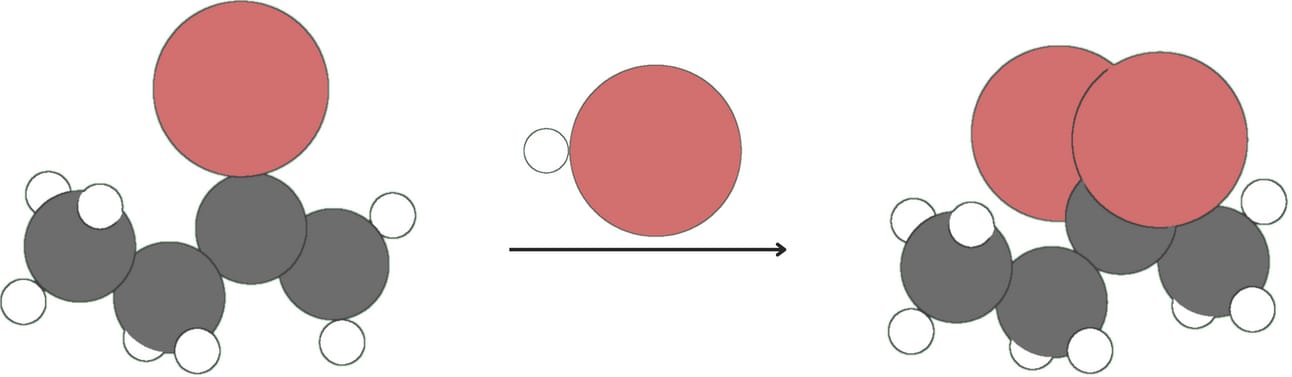
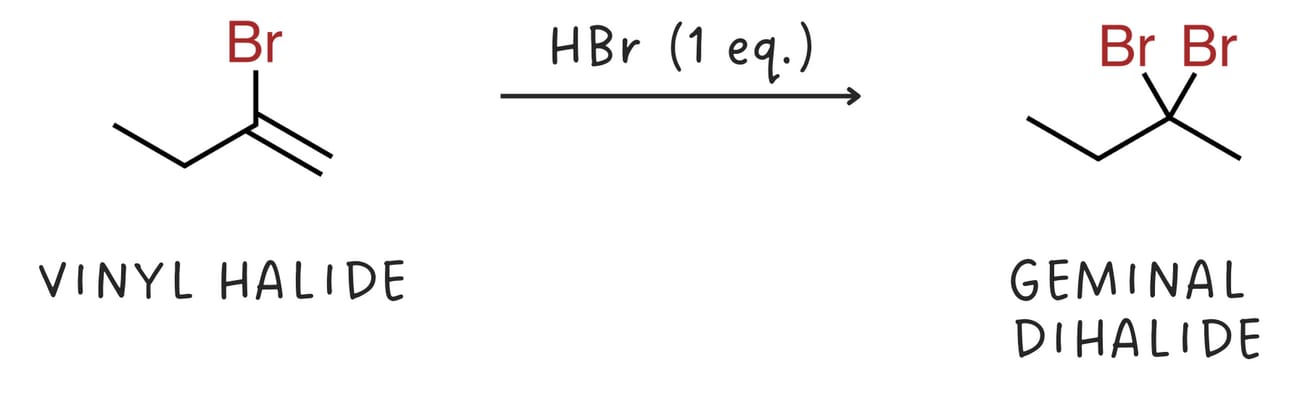
Alkynes react with one equivalent of hydrogen halide (HX, where X is a halogen like Cl or Br) to form vinyl halides.
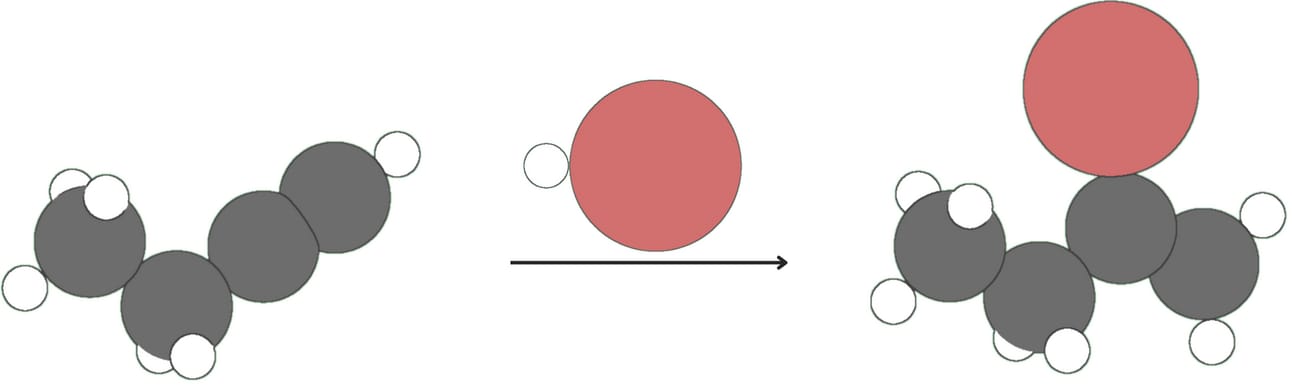
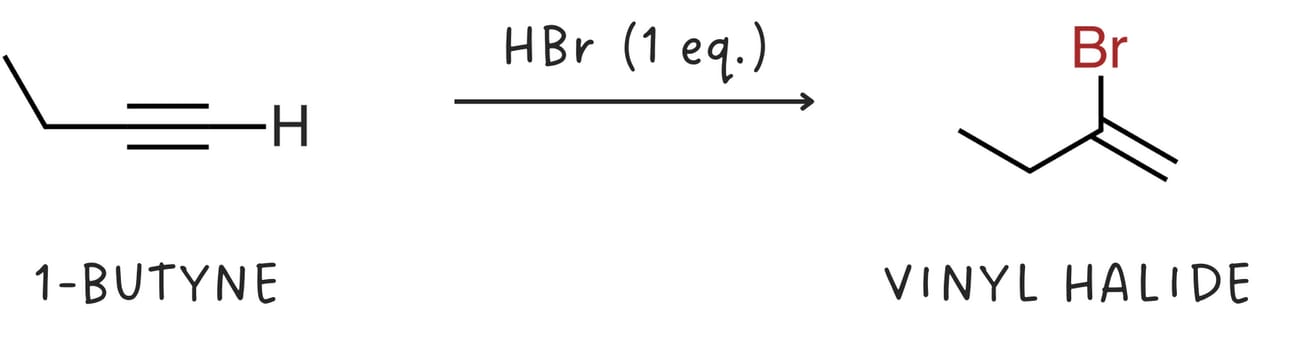
Vinyl halides can further react with HX to form geminal dihalides.
Hydroboration-oxidation (Addition of -H and -OH)
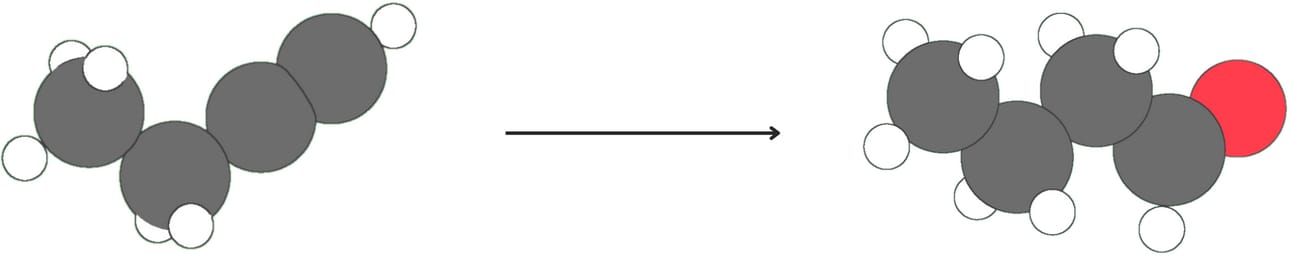
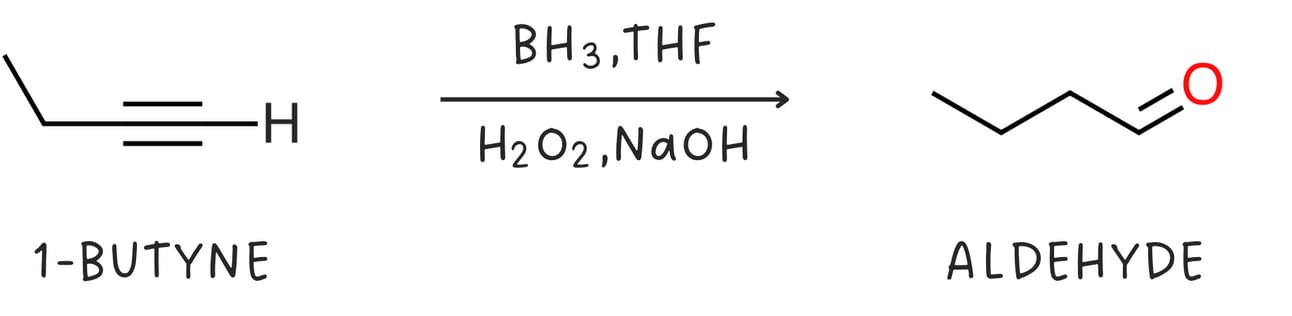
Regiochemistry: anti-Markovnikov
Like alkenes, alkynes undergo hydroboration-oxidation, which occurs with anti-Markovnikov regiochemistry as the -OH adds to the less substituted carbon. The initial enol product is unstable and quickly rearranges to form a more stable aldehyde (for terminal alkynes) or ketone (for internal alkynes)
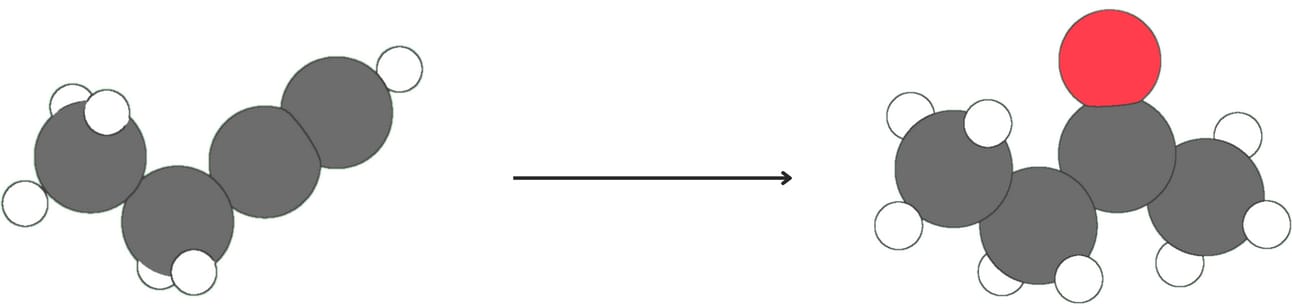
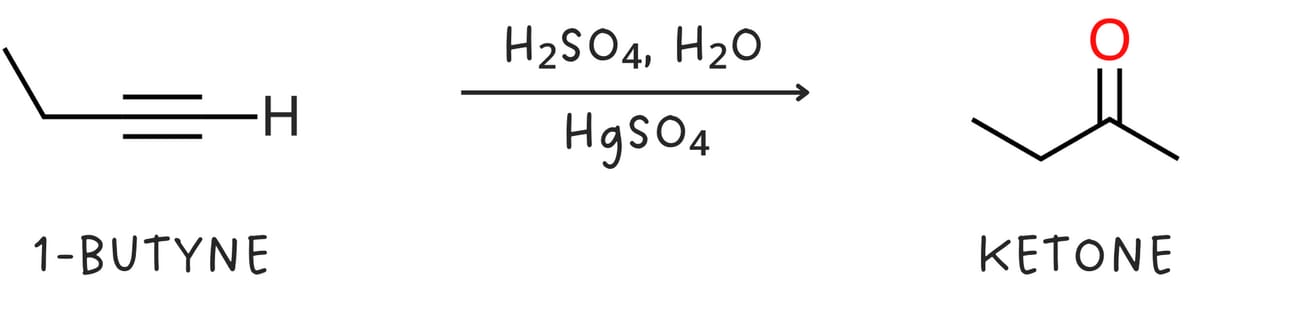
Mercury(II)-catalyzed hydration (Addition of -H and -OH)
Regiochemistry: Markovnikov
Unlike alkenes, alkynes react slowly with aqueous acid alone. Mercury(II) sulfate (HgSO4) is used as a catalyst to speed up the reaction. This reaction occurs with Markovnikov regiochemistry as the -OH group adds to the more substituted carbon. The initial enol product is unstable and quickly rearranges to form a more stable ketone.
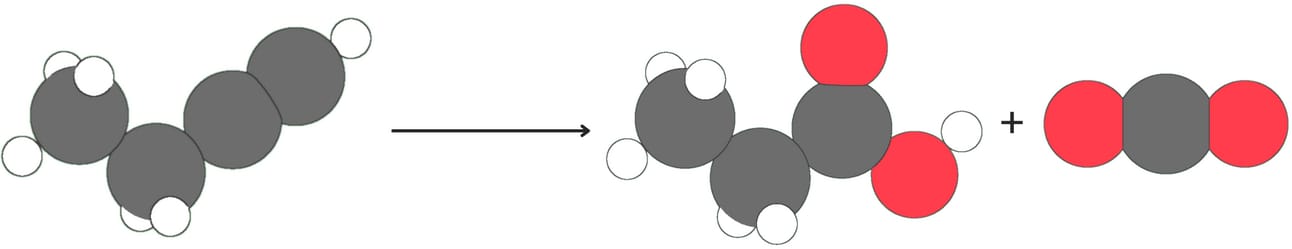
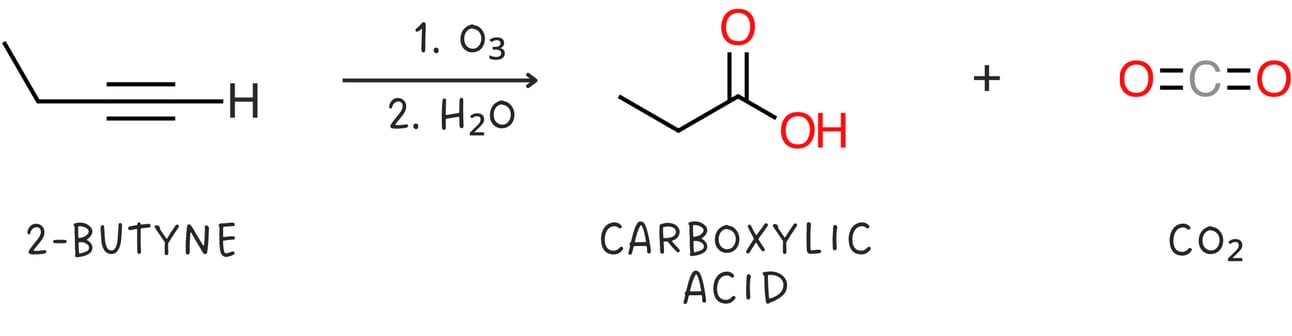
Ozonolysis
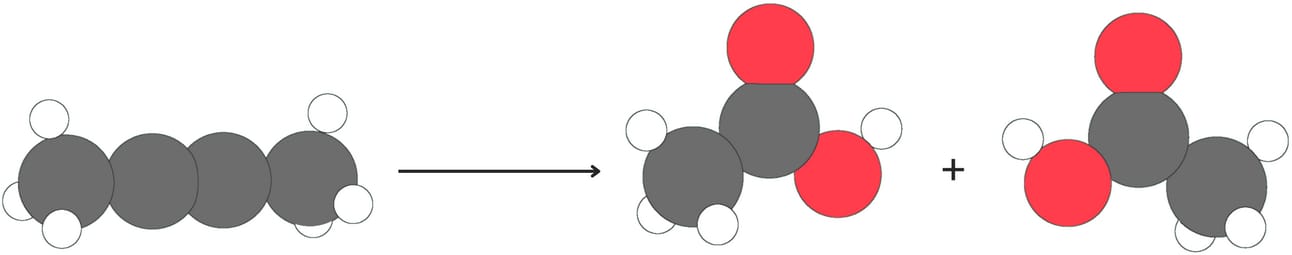
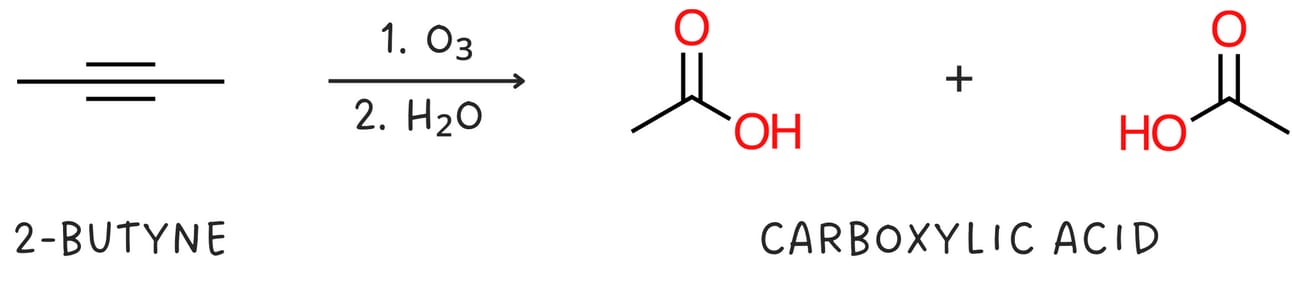
Alkynes can be cleaved when treated with oxidizing agents such as ozone or KMnO4 followed by H2O.
Internal alkynes produce two carboxylic acids
Terminal alkynes produce one carboxylic and CO2
Substitution reactions of alkynes
Terminal alkynes are unique due to their relatively high acidity compared to alkenes and alkanes.
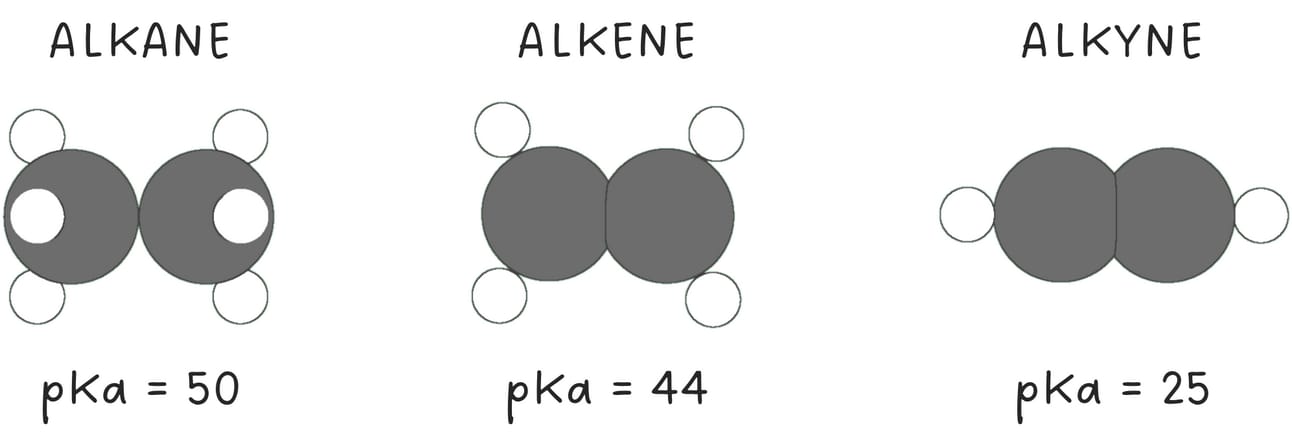
When terminal alkynes are treated with a strong base such as NaNH2, the terminal hydrogen is removed, forming an acetylide ion.
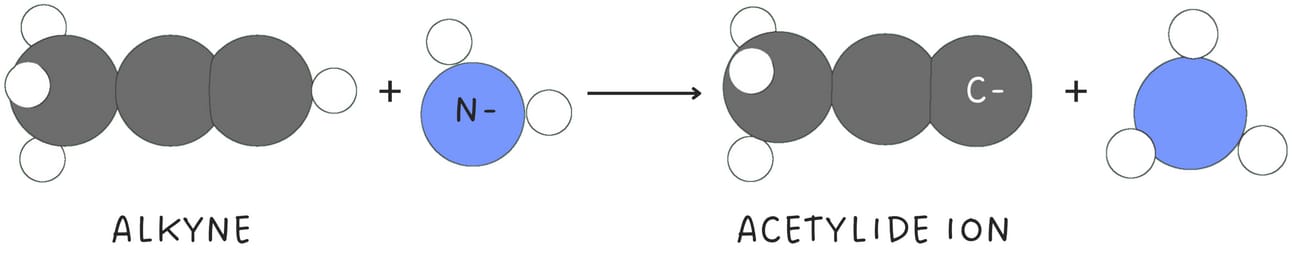
Acetylide ions are highly nucleophilic and function as nucleophiles in SN2 reactions, primarily with primary or methyl halides.
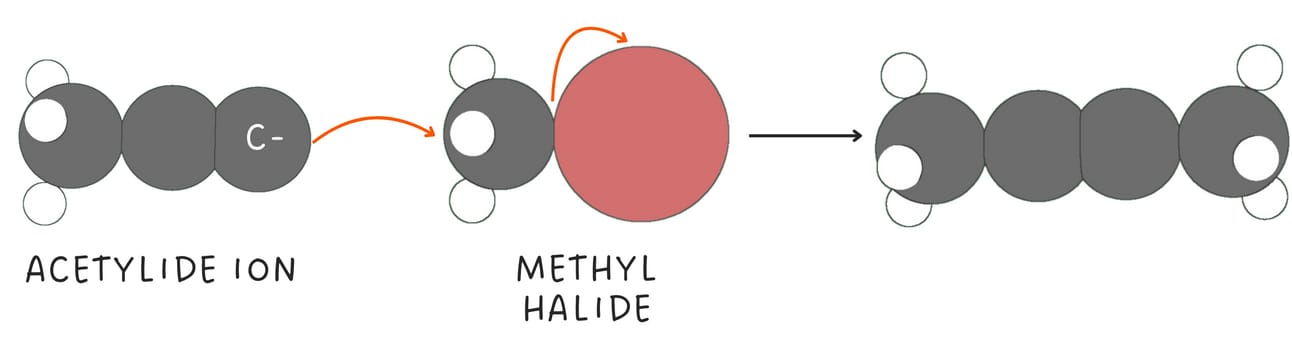
These reactions are called alkylation reactions and provide a versatile way to form C-C bonds and extend the length of a carbon chain.
Reply