- Scholarli
- Posts
- How to rank molecules by relative rate for SN1 reactions
How to rank molecules by relative rate for SN1 reactions
A step-by-step approach to ranking molecules in order of their expected SN1 reactivity by evaluating the stability of the resulting carbocation and the ability of the leaving group.
Objective
By the end of this guide, you should be able to rank molecules in order of their expected SN1 reactivity by evaluating the stability of the resulting carbocation and the ability of the leaving group.
🖐️ Before we start, make sure you're familiar with:
Substitution reactions
Characteristics of SN1 reactions
Let’s begin with a simple challenge.
Challenge 1: Ranking molecules with the same leaving group
Question:
Rank the following molecules in order of increasing relative rate for SN1 reaction (slowest to fastest reacting).

Here’s how we’ll do it.
Step 1: Identify the carbon that is bonded to the leaving group.
First, let's find the carbon atom that's bonded to the leaving group. Is it a methyl, primary (1°), secondary (2°), tertiary (3°), allylic, benzylic, or vinylic carbon?
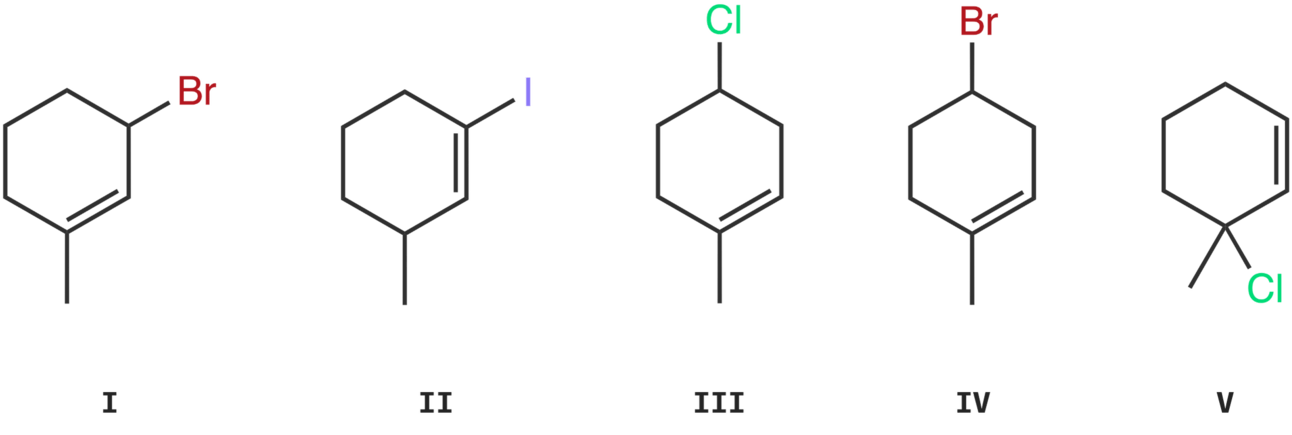
Molecule I
The leaving group, bromine, is bonded to a 1° carbon.

Molecule II
The leaving group, bromine, is bonded to a 2° allylic carbon.
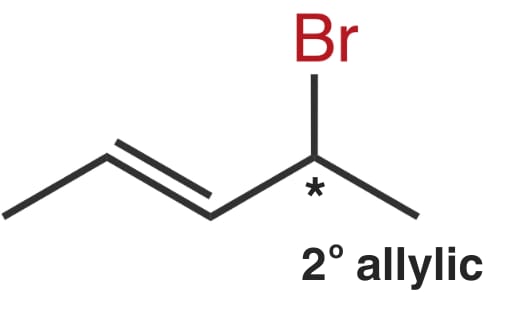
Molecule III
The leaving group, bromine, is bonded to a 1° allylic carbon.

Molecule IV
The leaving group, bromine, is bonded to a vinylic carbon.

This step is essential because it helps us determine the type of carbocation that will form after the leaving group departs.
In an SN1 reaction, the rate-determining step is the spontaneous loss of the leaving group, creating a carbocation.
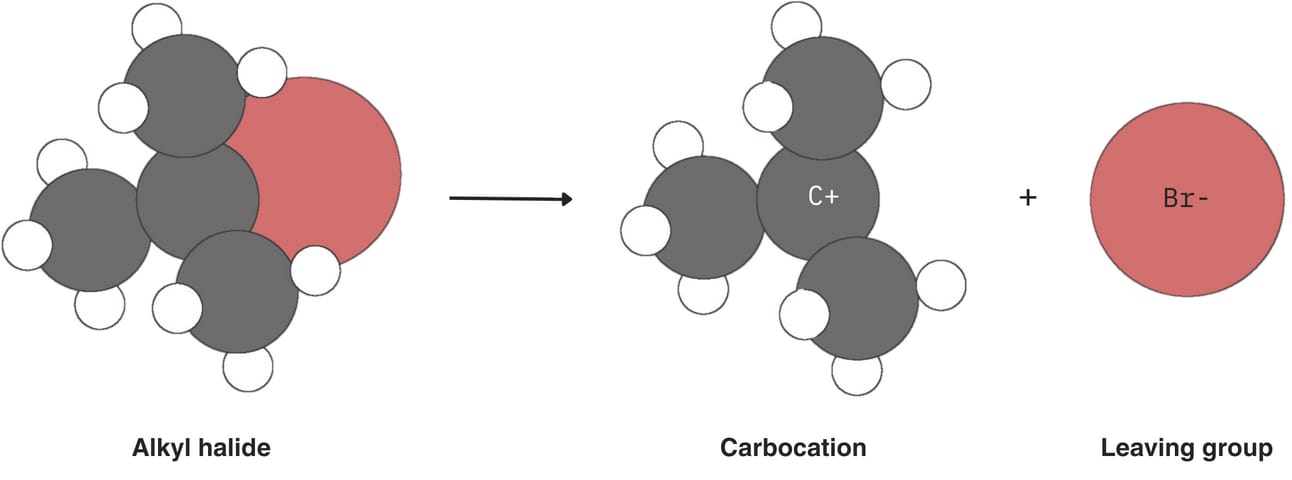
The stability of this carbocation is key: the more stable it is, the faster the reaction will proceed.
Now, let’s draw the carbocation for each molecule to analyze their stability.
Molecule I
Forms a 1° carbocation.

Molecule II
Forms a 2° allylic carbocation.

Molecule III
Forms a 1° allylic carbocation.

Molecule IV
In molecule IV, the leaving group is attached to a vinylic carbon. This means that the carbon bonded to the leaving group is part of a double bond.
Now, here’s an important point to remember: vinylic carbocations are highly unstable. Because of this instability, it is very unlikely for a vinylic carbocation to form during an SN1 reaction.

So, if you encounter a molecule with a leaving group on a vinylic carbon, you can confidently say that the SN1 reaction will be extremely slow or might not happen at all.
Step 2: Assess the leaving group
The best leaving groups are those that can stabilize the negative charge after departure. Generally, they are the conjugate bases of strong acids.
Recall that in the SN1 mechanism, the rate-determining step is the loss of the leaving group to form a carbocation. Hence, a good leaving group means a faster reaction.

All molecules in this example here have the same leaving group, bromine. This is not a determining factor.

In the next challenge, we will rank molecules where the leaving groups are different.
Step 3: Consider the solvent
Polar protic solvents such as water and alcohols stabilize the transition state and the carbocation intermediate, thus favoring SN1 reactions.
However, since no solvent information is provided in this question, we won't consider this factor for now.
Step 4: Combine carbocation stability and the leaving group's ability to determine the expected SN1 reactivity
Now, let's combine the knowledge of carbocation stability and the leaving group's ability.
Since all the molecules have the same leaving group, carbocation stability is the determining factor here.
Here's the stability order for carbocations:

Using this order, we can rank the molecules by their increasing relative rate for the SN1 reaction (from slowest to fastest reacting).

Molecule IV - Bromine on a vinylic carbon
Vinylic carbocations are highly unstable because the positive charge is on a carbon that is part of a double bond. This makes it very difficult for the carbocation to form, resulting in the slowest reaction rate.
Molecule I - Bromine on a 1° carbon
Primary carbocations are more stable than vinylic carbocations but are still quite unstable. This makes Molecule I react slowly, but faster than Molecule IV.
Molecule III - Bromine on a 1° allylic carbon
Allylic carbocations are stabilized by resonance, which means the positive charge can be delocalized over multiple atoms. Even though this is a primary carbocation, the allylic position provides extra stability, making Molecule III more reactive than Molecule I.
Molecule II - Bromine on a 2° allylic carbocation
Secondary carbocations are more stable than primary carbocations due to additional alkyl group stabilization. Additionally, being allylic means this carbocation benefits from resonance stabilization. This makes Molecule II the most reactive among the given molecules.
Final ranking from slowest to fastest SN1 reactivity:
IV < I < III < II
A quick note on nucleophiles
You might be wondering, what about the nucleophile?
In this case, we don't have any information about the nucleophile, but that's okay because the nucleophile doesn't affect the rate of an SN1 reaction.
Rate = k [alkyl halide]
This is because the carbocation intermediate formed during the reaction is highly reactive and will quickly react with any nucleophile that's present.
Challenge 2: Ranking molecules with different leaving groups
Alright, the first challenge was just the warm-up! Now, let’s dive into a more complex question, similar to what you might see on an actual organic chemistry exam.
Ready? Let’s go!
Sometimes, you might encounter a question that doesn't specify the type of reaction you're dealing with.
Question: Which alkyl halide will most rapidly undergo solvolysis in aqueous ethanol?
To tackle this question, remember that SN1 reactions are sometimes called solvolysis reactions.
What are solvolysis reactions, you ask?
Solvolysis reactions are a type of substitution reaction where the solvent acts as the nucleophile. In SN1 solvolysis reactions, the solvent (usually water or an alcohol) replaces the leaving group.
Step 1: Identify the carbon that is bonded to the leaving group.
First, let's find the carbon atom that's bonded to the leaving group. Is it a methyl, primary (1°), secondary (2°), tertiary (3°), allylic, or vinylic carbon?
Molecule I
The leaving group, bromine, is bonded to a 2° allylic carbon.
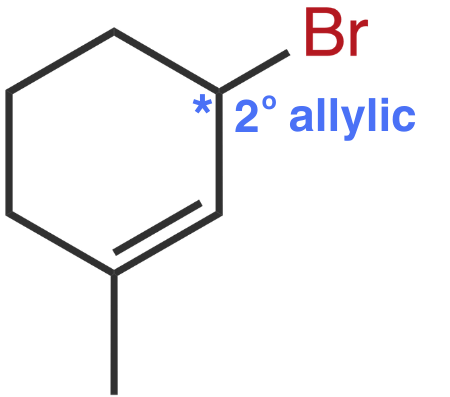
Molecule II
The leaving group, iodine, is bonded to a vinylic carbon.

Molecule III
The leaving group, chlorine, is bonded to a 2° carbon.
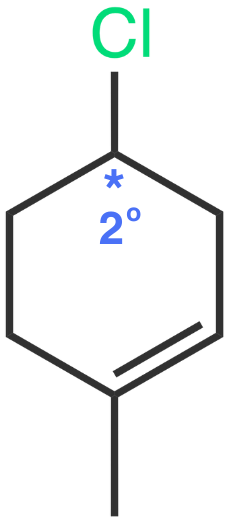
Molecule IV
The leaving group, bromine, is bonded to a 2° carbon.

Molecule V
The leaving group, chlorine, is bonded to a 3° allylic carbon.

Step 2: Assess the leaving group
Now, let’s evaluate the leaving groups. Remember, the best leaving groups are the most stable anions, typically the conjugate bases of strong acids.

Let's look at the leaving groups in our molecules:
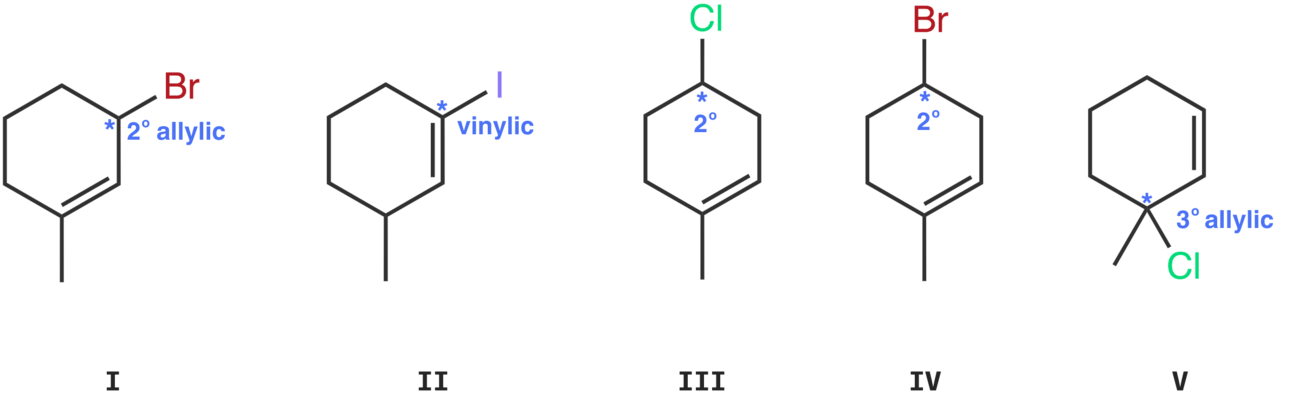
Molecule I - Bromine on a 2° allylic carbon
Molecule II - Iodine on a vinylic carbon
Molecule III - Chlorine on a 2° carbon
Molecule IV - Bromine on a 2° carbon
Molecule V - Chlorine on a 3° allylic carbon
When comparing Molecules III and IV, we see that both have their leaving groups bonded to 2° carbons. However, bromine is a better leaving group than chlorine, so Molecule IV will react faster than Molecule III.
Step 3: Consider the solvent
In this question, the solvent provided is aqueous ethanol.
Since we are comparing the relative rates of each molecule in the same solvent, the solvent is not a determining factor for this specific comparison.
By focusing on the carbocation stability and the ability of the leaving group, we can accurately rank the reactivity of the molecules without worrying about the solvent in this case. This keeps things simple and allows us to concentrate on the key factors that affect the reaction rate.
Keep this in mind as you practice, and you'll become more confident in predicting reaction rates!
Step 4: Combine carbocation stability and the leaving group's ability to determine the expected SN1 reactivity
Now, let's combine what we know about carbocation stability and the ability of the leaving group.
Here's the stability order for carbocations:

Based on the stability order for carbocations and the ability of the leaving group, we can rank these molecules from slowest to fastest SN1 reactivity as follows:

Molecule II - Iodine on a vinylic carbon
Vinylic carbocations are highly unstable because the positive charge is on a carbon that is part of a double bond. This makes it very difficult for the carbocation to form, resulting in the slowest reaction rate.
Molecule III - Chlorine on a 2° carbon
Secondary carbocations are more stable than primary but less stable than tertiary. Chlorine is a decent leaving group but not as good as bromine. Therefore, this molecule will have a slower reaction rate compared to those with bromine as the leaving group.
Molecule IV - Bromine on a 2° carbon
Like Molecule III, this forms a secondary carbocation. However, bromine is a better leaving group than chlorine, making the reaction faster than Molecule III.
Molecule I - Bromine on a 2° allylic carbon
Allylic carbocations are stabilized by resonance, making them more stable than regular secondary carbocations. With bromine as a good leaving group, this molecule will react faster than Molecules III and IV.
Molecule V - Chlorine on a 3° allylic carbon
Tertiary allylic carbocations are highly stable due to both hyperconjugation and resonance effects. Even though chlorine is not as good a leaving group as bromine, the stability of the tertiary allylic carbocation makes this the fastest reacting molecule.
Final ranking from slowest to fastest SN1 reactivity:
II < III < IV < I < V
Great job! By understanding carbocation stability and the abilities of different leaving groups, you've successfully ranked the molecules by their expected SN1 reactivity.
Keep practicing, and these concepts will soon become second nature! Ready for the next challenge? Let's go!
Challenge 3
Now, let’s tackle another challenging question.
Question: Rank the following molecules in order of increasing reaction rate with methanol.

This question might seem a bit tricky because it doesn’t specify the type of reaction. It doesn’t mention ‘solvolysis’ or ‘SN1 reaction’. But don’t worry, we’ve got this!
First, let's look at the key information provided: the molecules are alkyl halides, and methanol is involved. Methanol is a weak nucleophile and a common polar protic solvent. So, we know that it’s most likely a substitution reaction. But the question is, which one: SN1 or SN2?
Here’s the clue: weak nucleophiles and polar protic solvents favor SN1 reactions. Therefore, we can infer that this reaction is likely an SN1 reaction.
Step 1: Identify the carbon that is bonded to the leaving group.
First, let's find the carbon atom that's bonded to the leaving group. Is it a methyl, primary (1°), secondary (2°), tertiary (3°), allylic, or vinylic carbon?
Molecule I
The leaving group, bromine, is bonded to a 1° allylic carbon.

Molecule II
The leaving group, bromine, is bonded to a 1° carbon.

Molecule III
The leaving group, bromine, is bonded to a 2° allylic carbon.
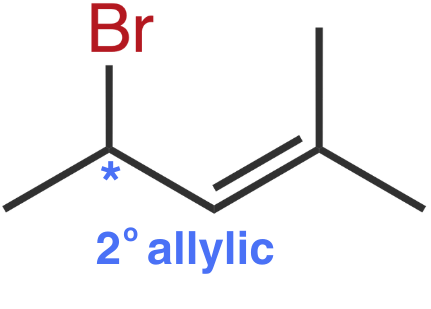
Molecule IV
The leaving group, bromine, is bonded to a 2° allylic carbon.
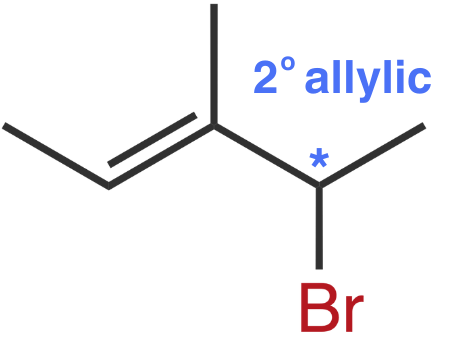
Molecule V
The leaving group, bromine is bonded to a vinylic carbon.

Step 2: Assess the leaving group
All molecules in this example here have the same leaving group, bromine. This is not a determining factor.
Step 3: Consider the solvent
In this question, the solvent provided is methanol.
Since we are comparing the relative rates of each molecule in the same solvent, the solvent is not a determining factor for this specific comparison.
Step 4: Combine carbocation stability and the leaving group's ability to determine the expected SN1 reactivity
Since all molecules have the leaving group bromine, the deciding factor here is the stability of the carbocation formed.
Here's the stability order for carbocations:

Based on the stability order for carbocations, we can rank these molecules from slowest to fastest SN1 reactivity as follows:

V (vinylic) < II (1°) < I (1° allylic) < ? < ?
Now, what about Molecules III and IV?
Both Molecules III and IV have leaving groups bonded to 2° allylic carbons. To determine which one reacts the fastest, we need to consider the resonance forms of the carbocations they form.
Molecule III
Molecule III forms a 2° allylic carbocation.

The positive charge is delocalized over a 2° and a 3° carbon. This carbocation benefits from hyperconjugation provided by the tertiary carbon.

Molecule IV
Molecule IV also forms a 2° allylic carbocation.

In Molecule IV’s carbocation, the positive charge is delocalized over two 2° carbons. While still stabilized by resonance, it doesn't benefit from the additional stability of a tertiary position.

Therefore, Molecule III will react faster than Molecule IV due to the additional stability provided by the tertiary carbon.
V < II < I < III < IV
Great job! By understanding carbocation stability and the effects of resonance, you've successfully ranked the molecules by their expected SN1 reactivity.
Keep practicing, and these concepts will soon become second nature!
Reply