- Scholarli
- Posts
- How to draw a mechanism for a Brønsted-Lowry acid-base reaction
How to draw a mechanism for a Brønsted-Lowry acid-base reaction
A step-by-step guide to drawing a mechanism and predicting the products of a Brønsted-Lowry acid-base reaction
Objective
Welcome to our guide! By the end of this journey, you'll be able to predict the products of acid-base reactions using the Brønsted-Lowry theory. You'll also be able to identify the acid and base in a reaction, draw curved arrows to show the flow of electrons, and label the conjugate acid-base pairs.
🖐️ Before we start, make sure you're familiar with:
Brønsted-Lowry acid-base theory
Common acids and bases
Let’s begin with a simple challenge.
Challenge 1
Question:
Draw a mechanism for the following acid-base reaction. Label the acid, base, conjugate acid, and conjugate base.

Here’s how we’ll do it:
Step 1: Identify the acid and the base
In this reaction, water donates a proton to form hydroxide, making water the acid.
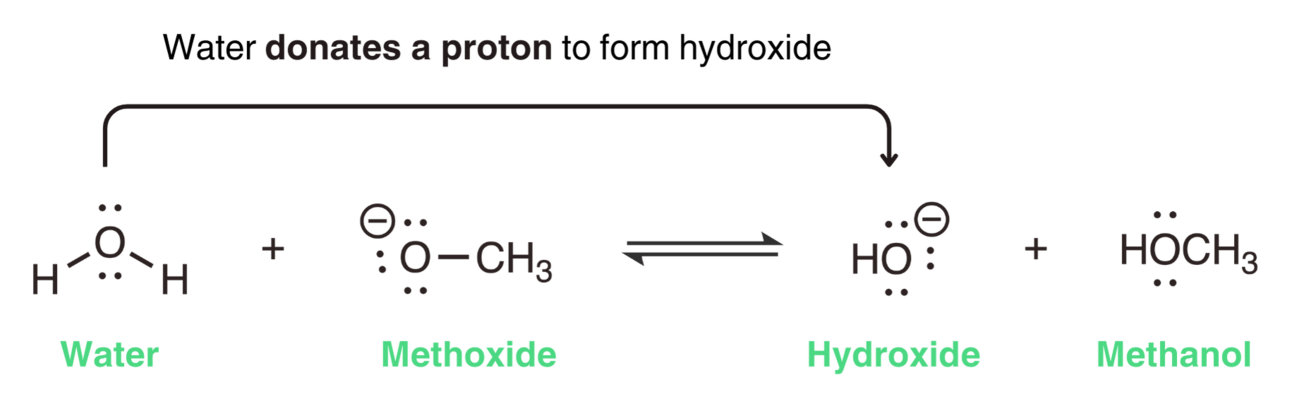
Methoxide accepts the proton to form methanol, making methoxide the base.

Step 2: Draw the curved arrows to show the flow of electrons
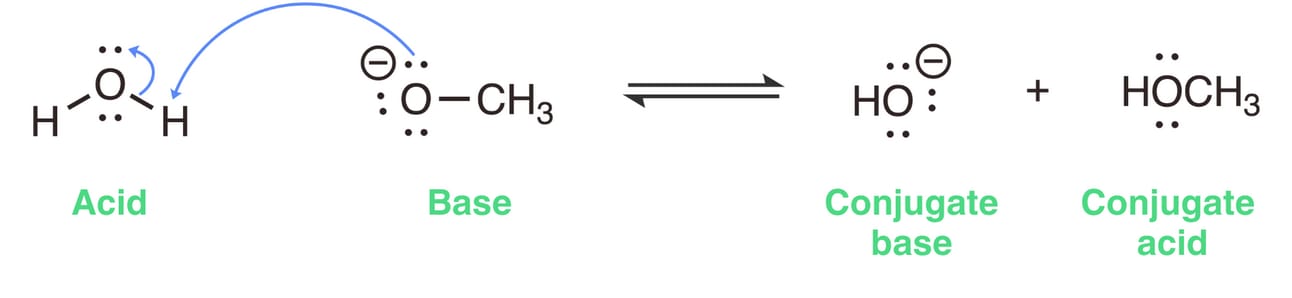
To draw the mechanism of this acid-base reaction, we use two curved arrows.
We draw the first curved arrow from a lone pair on the base to a proton on the acid. This indicates that methoxide is donating a pair of electrons (two electrons) to the hydrogen atom.

Since hydrogen can only hold two electrons, another electron pair must move away from hydrogen. This breaks the H-O bond, and the electrons remain with the oxygen atom.
So, draw a second curved arrow from the center of the H-O bond in water to the oxygen atom.

Step 3: Identify the conjugate acid-base pairs
In this reaction, water (H2O) donates a proton to become hydroxide (OH- ), making hydroxide the conjugate base. Methoxide (OCH3- ) accepts a proton to become methanol (CH3OH), making methanol the conjugate acid.
Great job! Let's take it up a notch with the next challenge.
Most times on your exam, you will be asked to draw a mechanism AND predict the products of an acid-base reaction.
Challenge 2
Question:
Predict the products and draw a mechanism for the following acid-base reaction. Label the acid, base, conjugate acid, and conjugate base.

Step 1: Identify the acid and the base
Unlike the previous example, we don’t have the products provided, so let's identify the acid and base by their charges.
Bases are typically negatively charged or neutral, with negatively charged bases stronger than their neutral counterparts.
Acids are usually neutral or positively charged, with positively charged acids stronger than their neutral counterparts.
Let’s start by identifying all lone pairs, dipoles, and charges.
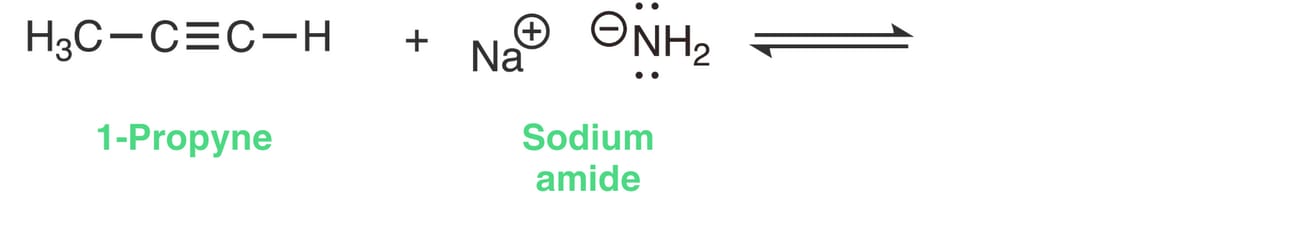
NaNH2 contains an ionic bond: Na⁺ and NH₂⁻
Na+ is a spectator ion and does not participate in the reaction.
NH2- is negatively charged and will function as the base (proton acceptor).
1-Propyne will function as the acid (proton donor).

Step 2: Draw curved arrows to show the flow of electrons
NH2- acting as the base will accept a proton from 1-propyne.

🗒️ Note
1-Propyne has two different sets of protons, each bonded to carbons with distinct hybridizations. Let's break down their differences.

Proton a is bonded to an sp³ hybridized carbon.
Proton b is bonded to an sp hybridized carbon.
Protons bonded to sp hybridized carbons, like proton b, are more acidic than those bonded to sp³ hybridized carbons, like proton a. This increased acidity is due to the higher s-character in sp hybridized carbons, which means the electrons are held closer to the nucleus, stabilizing the negative charge more effectively when the proton is removed.
Since proton b is more acidic, it is more likely to be donated during the reaction with NH₂-.
Draw an arrow from a lone pair on NH2- to the most acidic proton on 1-propyne.
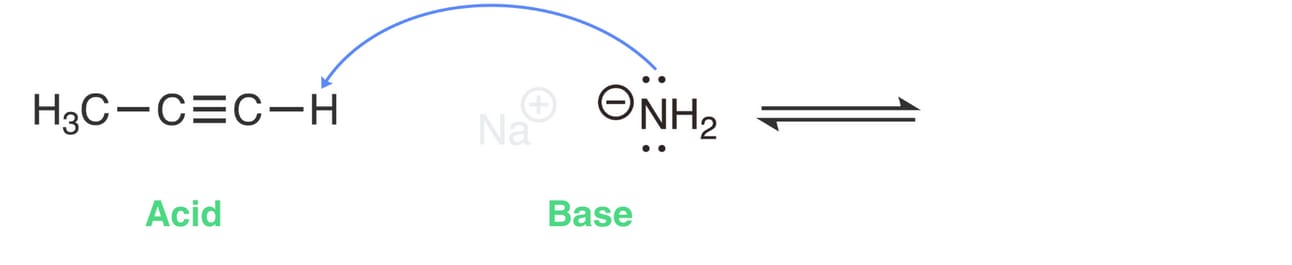
Since hydrogen can only hold two electrons, another electron pair must move away from hydrogen. This breaks the H-C bond, and the electrons remain with the carbon atom.
So, draw a second curved arrow from the center of the H-C bond in 1-propyne to the carbon atom.

Step 3: Identify the conjugate acid-base pairs


Now that we have predicted the products of this reaction, let's write the equation for the reaction and include the curved arrow mechanism to illustrate the electron flow.

Great work! By following these steps, you have successfully identified the acid and base, shown the electron flow with curved arrows, identified and labeled the conjugate acid-base pairs, and written the reaction equation.
Ready for more practice? Let’s keep going with another challenge!
Reply