- Scholarli
- Posts
- Acids and bases: A summary
Acids and bases: A summary
A comprehensive summary of acids and bases in Organic Chemistry 1

Table of Contents
Brønsted-Lowry Acid-Base Theory
Definition
The Brønsted-Lowry theory describes acidity and basicity in terms of proton transfer. A Brønsted-Lowry acid (HA) is a proton donor, while a Brønsted-Lowry base is a proton acceptor.

A Brønsted-Lowry acid-base reaction produces a conjugate acid and a conjugate base (A-).
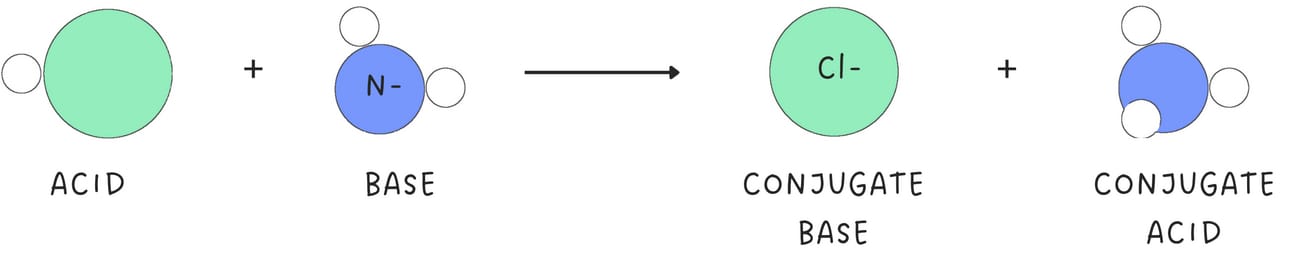
A conjugate base typically bears a negative charge.

The relative strength of an acid can be predicted by analyzing the stability of the negative charge on its conjugate base.
If A- is very stable, then HA will be willing to give up the proton; therefore, HA must be a strong acid.
If A- is very unstable, HA must be a weak acid.
Factors that affect the stability of a negative charge
Atom bearing the charge: What type of atom holds the negative charge?
Resonance: Can the charge be distributed over multiple atoms?
Inductive effects: Do nearby electronegative atoms help stabilize the charge?
Orbitals: How close is the orbital the charge occupies to the nucleus?
Atom bearing the charge
For atoms in the same row of the periodic table, electronegativity is the dominant effect. More electronegative atoms are willing to accept electrons and therefore stabilize a negative charge.
Therefore, across the periodic table, acidity increases with electronegativity.

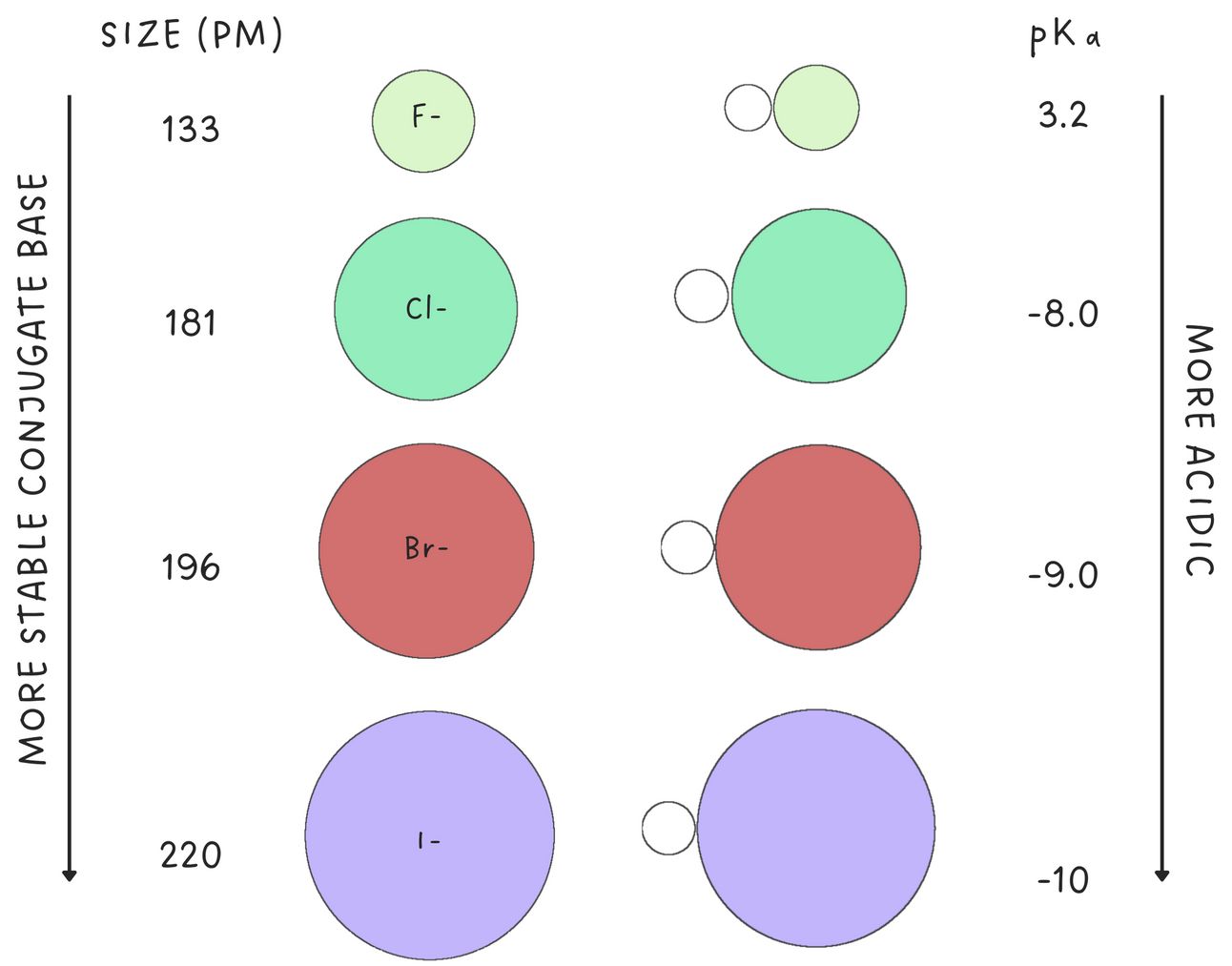
For atoms in the same column of the periodic table, size is the dominant effect. Larger atoms are more capable of stabilizing a negative charge because the charge is spread over a larger volume of space.
Therefore, down the periodic table, acidity increases with size.
Resonance
Resonance stabilizes a negative charge by allowing it to be spread over more than one atom.

Inductive effect
Electron-withdrawing groups, such as halogens, stabilize a nearby negative charge by “pulling” electron density to themselves. There are three factors to consider when dealing with inductive effect.
Acidity increases with the number of electronegative atoms

Acidity increases with increasing electronegativity

Acidity increases with decreasing distance from the negative charge

The opposite of inductive effect is hyperconjugation. Electron-donating groups, such as alkyl groups, destabilize a nearby negative charge.
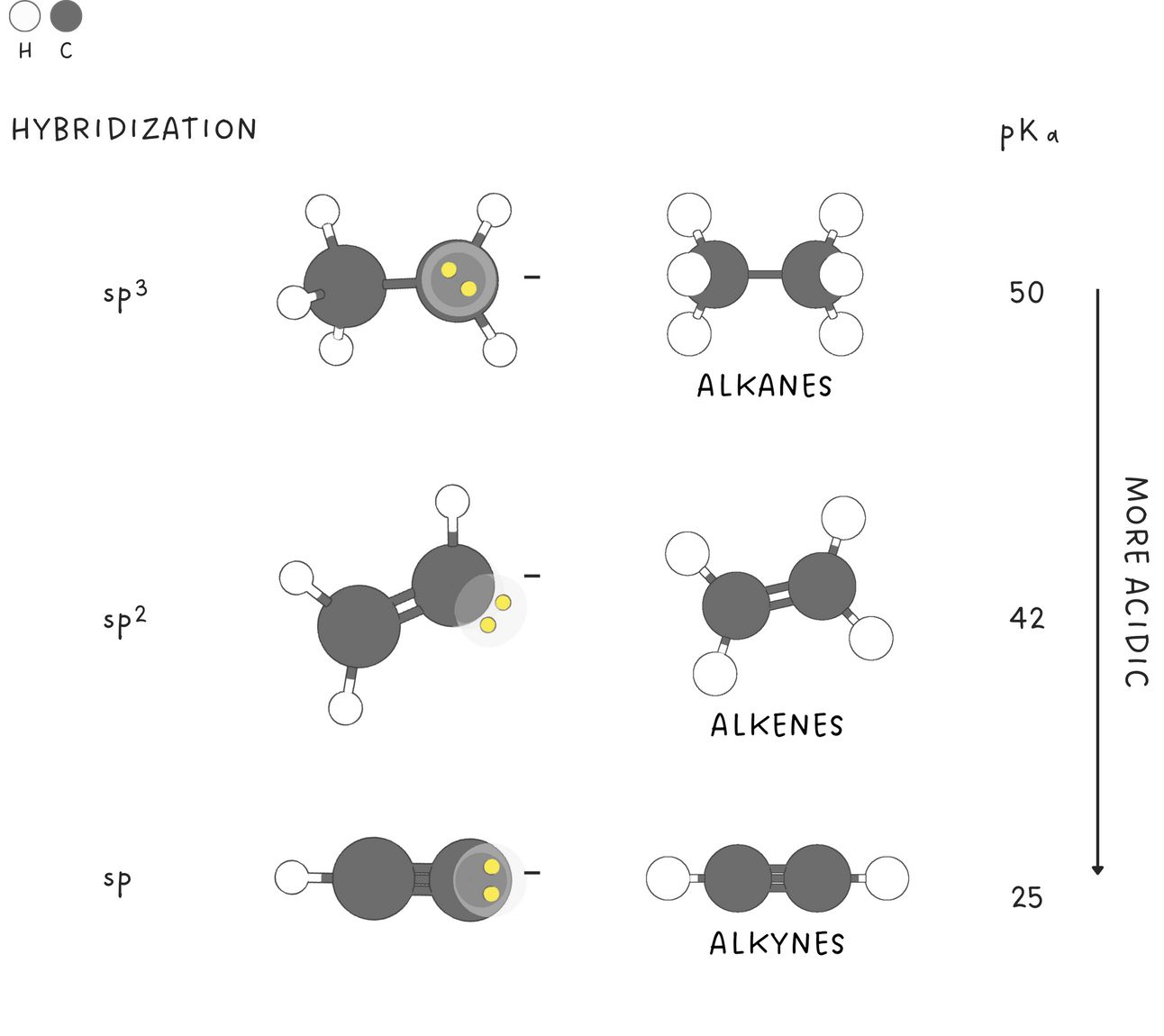
Orbitals
A negative charge in an sp-hybridized orbital is closer to the positively charged nucleus and more stable than a negative charge in an sp3 -hybridized orbital. Therefore,
Predicting the position of equilibrium
The equilibrium of an acid-base reaction always favors whichever side has the more stable base.

Lewis Acid-Base Theory
The Lewis theory describes acidity and basicity in terms of electron transfer. A Lewis acid is an electron pair acceptor, while a Lewis base is an electron pair donor.

Lewis acids are known as electrophiles while Lewis bases are known as nucleophiles.

All Brønsted-Lowry acids and bases can be classified as Lewis acids and bases.

Reaction Mechanisms
We use curved arrows to show how electrons flow in a reaction. In drawing reaction mechanisms, the curved arrows point from the nucleophile, the source of electrons, to the electrophile, the electron acceptor.
Proton transfer

Nucleophilic attack

Identifying a nucleophile
Nucleophiles contain electron-rich centers. These electron-rich centers can be atoms with lone pairs, pi bonds, or sigma bonds. Nucleophiles can be neutral or negatively charged.
Lone pairs

Pi bonds

Sigma bonds

Identifying an electrophile
Electrophiles contain electron-poor centers. Electron-poor centers include atoms with empty orbitals and atoms that bear a partial positive charge due to induction or polarization. Electrophiles can be positively charged or neutral.
Empty orbitals

Partial positive charge

Reply